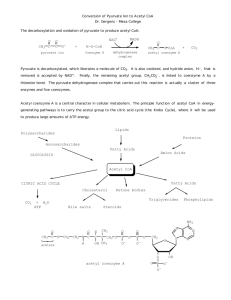
CH16-18 1. Describe the general structure , regulation and required cofactors/vitamins of the pyruvate dehydrogenase complex. List the differences between it and the alpha-ketoglutarate dehydrogenase. 2. With the given descriptions, draw the arrow pushing mechanism for the PDH complex: A. Decarboxylation: TPP is combined with the pyruvate and decarboxylated in order to yield hydroxyethyl-TPP. Of the pyruvate dehydrogenase component, TPP is known as the prosthetic group which the carbon atom between the nitrogen and sulfur atoms in the thizaole ring is more acidic than most double bonded carbon groups with pKa values near 10. This reaction is catalyzed by the (E1) pyruvate dehydrogenase component of the multienzyme complex. The carbon center located in the TPP is ionized to form a carbanion which is added to the carbonyl group of pyruvate. As part of decarboxylation, a positive charged ring of TPP stabilizes the negative charge which was transferred to the ring. Finally, the protonation yields hydroxyethyl-TPP. B. Oxidation: In order to form an acetyl group, the hydroxyethyl group which is attached to TTP is oxidized. Simultaneously, the hydroxyethyl group is transferred to lipoamide which is lipoic acid derived that links to the side chain of a lysine residue by an amide linkage. This creates the formation of an energy-rich thioester bond. In this reaction, the disulfide group of lipoamide acts as an oxidant and is reduced to the disulfhydryl form. This reaction is catalyzed by the pyruvate dehydrogenase component (E1) as well and yields the acetyllipoamide. C. Formation of Acetyl CoA: Formation of Acetyl CoA: In this step, acetyl CoA is formed when acetyl group is transferred from acetyllipoamide. This reaction is catalyzed by dihydrolipoyl transacetylase (E2). As the acetyl group is transferred to the CoA, the energy-rich thioester bond is preserved. Thus, the fuel for the citric acid cycle, acetyl CoA has been generated from pyruvate for use. Until the dihydrolipoamide is oxidized to lipoamide, the pyruvate dehydrogenase complex cannot complete another catalytic cycle. D. Formation of NADH: The final step in this reaction occurs when the oxidized form of lipoamide is regenerated by dihydrolipoyl dehydrogenase (E3).Two electrons are transferred to first an FAD prosthetic group of the enzyme and then to NAD+. This process of transferring electron is very unusual because FAD are known to receive electrons from NADH, not transfer them. Within the enzyme, the electrontransfer potential of FAD is increased by its chemical environment which enables it to transfer electrons to NAD+. Flavoproteins are proteins which are tightly associated with FAD or FMN also known as flavin mononucleotide. 3. Describe the reaction catalyzed by citrate synthesis, including reactants, intermediates, products free energy change and regulation. 4. List the enzymes of the TCA cycle that catalyze redox reactions or substrate level phosphorelation reactions 5. What are the anaplerotic reactions? What prosthetic group is common to enzymes that catalyze anaplerotic reactions? 6. In the presence of saturating amounts of oxaloacetate, the activity of citrate synthase from pig heart tissue shows a sigmoid dependence on the concentration of acetyl-CoA, as shown in the graph. When succinyl-CoA is added, the curve shifts to the right and the sigmoid dependence is more pronounced. On the basis of these observations, suggest how succinyl-CoA regulates the activity of citrate synthase. (Hint: See Fig. 6-34.) Why is succinyl-CoA an appropriate signal for regulation of the citric acid cycle? How does the regulation of citrate synthase control the rate of cellular respiration in pig heart tissue? 7.Describe the absorption and distribution of dietary lipids: 8. Describe the reactions for the enzymes involved in the beta oxidation ofn fatty acids (in terms of reactants, products enzymes, reaction type, and coenzymes required: 9. Describe the transport and regulation of fatty acids into the mitochondria for beta oxidation: 10. Describe the differences in beta oxidation of saturated vs unsaturated fatty acids 11. If [3-14C] propionate (14C in the methyl group) is added to a liver homogenate, 14C-labeled oxaloacetate is rapidly produced. Draw a flow chart for the pathway by which propionate is transformed to oxaloacetate, and indicate the location of the 14C in oxaloacetate 12. List the ketone bodies formed in the body, and why are they prfered rather than fatty acids? 13. Describe the type of reaction and the coenzyme required for the first stem in amino acid catabolism: 14. Describe the reactions of the Urea Cycle, including the (A) specific enzymes, (B) the input substrates (NH4, HCO3, ornithine, and aspartate), (C) connections to the TCA cycle, and (D) energy requirements. 15. Describe the regulation of the Urea cycle: