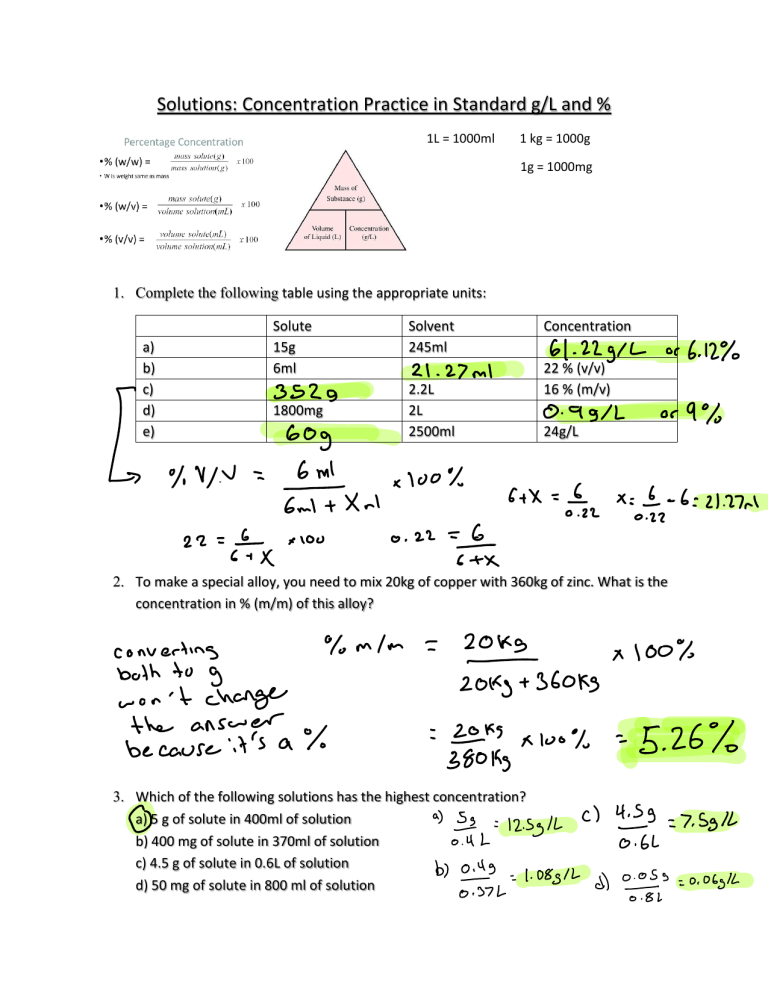
Solutions: Concentration Practice in Standard g/L and % 1L = 1000ml 1 kg = 1000g 1g = 1000mg 1. Complete the following table using the appropriate units: a) b) c) d) e) Solute 15g 6ml Solvent 245ml Concentration 3529 2.2L 2L 2500ml 21.27 ml 22 % (v/v) 16 % (m/v) 1800mg 60g You 22 644 fix too 61.22g 0.991L or 6.12 or 24g/L x 00 É 22 42 o 22 L 9 6 21.27 2. To make a special alloy, you need to mix 20kg of copper with 360kg of zinc. What is the concentration in % (m/m) of this alloy? both to g mlm Fff won't change answer the É because it's a 31 s go 5.26 100 3. Which of the following solutions has the highest concentration? a) 5 g of solute in 400ml of solution 12 b) 400 mg of solute in 370ml of solution c) 4.5 g of solute in 0.6L of solution d) 50 mg of solute in 800 ml of solution Sf 100 sk c imma 4 1 7.5812 4. If you make some iced tea from a powder mix and the instructions tell you to mix 15g of powder with 400ml of water, what is the concentration of the sugary drink in g/L? What about in % (m/v)? 6 41 37.5912 3.75 or alloy 5. What do you call a solid solution? ____________________ 6. What volume in ml would you need to add to 36g of Kool aide to make a drink with a concentration of 150g/L? re si o 7. Pepsi has a sugar concentration of 9.8 % (m/v). How many g of sugar are in a 355ml can of Pepsi? 98 100 g 0.0985 0.098 a 355 1 34.79g 8. How many grams of protein powder would you need to make a protein shake with a concentration of 40g/L in a 750ml cup of water? Substance being dissolved É 4051L x 0.75 Salt in salt water 9. What is a solute? __________________ Can you give an example? ________________ Water inssalt water Substance doing you give an example? __________________ What is ain solvent? _________________Can the dissolving