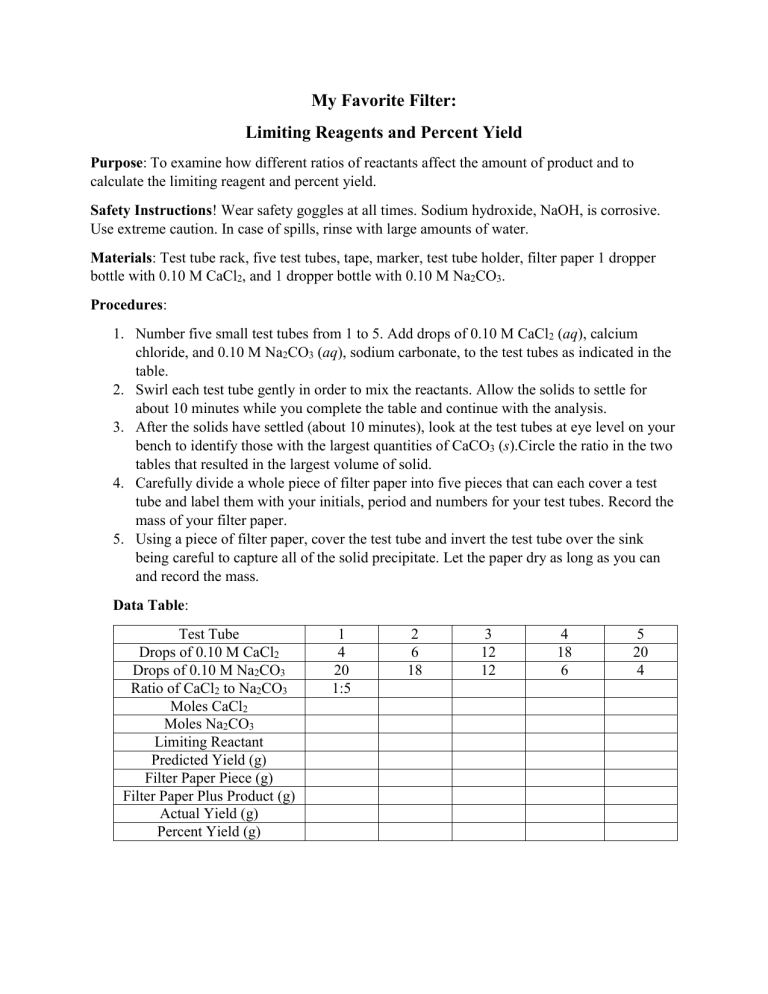
My Favorite Filter: Limiting Reagents and Percent Yield Purpose: To examine how different ratios of reactants affect the amount of product and to calculate the limiting reagent and percent yield. Safety Instructions! Wear safety goggles at all times. Sodium hydroxide, NaOH, is corrosive. Use extreme caution. In case of spills, rinse with large amounts of water. Materials: Test tube rack, five test tubes, tape, marker, test tube holder, filter paper 1 dropper bottle with 0.10 M CaCl2, and 1 dropper bottle with 0.10 M Na2CO3. Procedures: 1. Number five small test tubes from 1 to 5. Add drops of 0.10 M CaCl2 (aq), calcium chloride, and 0.10 M Na2CO3 (aq), sodium carbonate, to the test tubes as indicated in the table. 2. Swirl each test tube gently in order to mix the reactants. Allow the solids to settle for about 10 minutes while you complete the table and continue with the analysis. 3. After the solids have settled (about 10 minutes), look at the test tubes at eye level on your bench to identify those with the largest quantities of CaCO3 (s).Circle the ratio in the two tables that resulted in the largest volume of solid. 4. Carefully divide a whole piece of filter paper into five pieces that can each cover a test tube and label them with your initials, period and numbers for your test tubes. Record the mass of your filter paper. 5. Using a piece of filter paper, cover the test tube and invert the test tube over the sink being careful to capture all of the solid precipitate. Let the paper dry as long as you can and record the mass. Data Table: Test Tube Drops of 0.10 M CaCl2 Drops of 0.10 M Na2CO3 Ratio of CaCl2 to Na2CO3 Moles CaCl2 Moles Na2CO3 Limiting Reactant Predicted Yield (g) Filter Paper Piece (g) Filter Paper Plus Product (g) Actual Yield (g) Percent Yield (g) 1 4 20 1:5 2 6 18 3 12 12 4 18 6 5 20 4 Analysis: 1. Write the balanced chemical equation for the reaction of aqueous calcium chloride with aqueous sodium carbonate to produce calcium carbonate precipitate in an aqueous sodium chloride solution. 2. Use the balanced chemical equation to predict the ratio of moles of CaCl2 (aq) and Na2CO3 (aq) that will produce the maximum amount of CaCO3 (s). 3. Calculate the moles of CaCl2 and moles of Na2CO3 and determine the limiting reactant for each test tube. 4. Calculate the predicted yield of CaCO3 (s) for each test tube. 5. Calculate the percent yield for each test tube. 6. How do the balanced chemical equations compare to your observations? 7. Suppose you mix calcium chloride, CaCl2 (aq), with sodium oleate, NaC18H33O2 (aq). What products do you expect? Assuming the two solutions have the same concentration, what ratio of drops would give you the largest amount of precipitate? (Note: One product is calcium oleate, commonly referred to as soap scum.) 8. How do you determine the limiting reagent of a reaction? (Involve the mole ratio.) 9. How do you determine the percent yield of a reaction? What calculation is similar? Error Analysis: Write a four sentence error analysis about your accuracy (percent yield), precision (consistency of your percent yield) and errors (random, systemic, human).