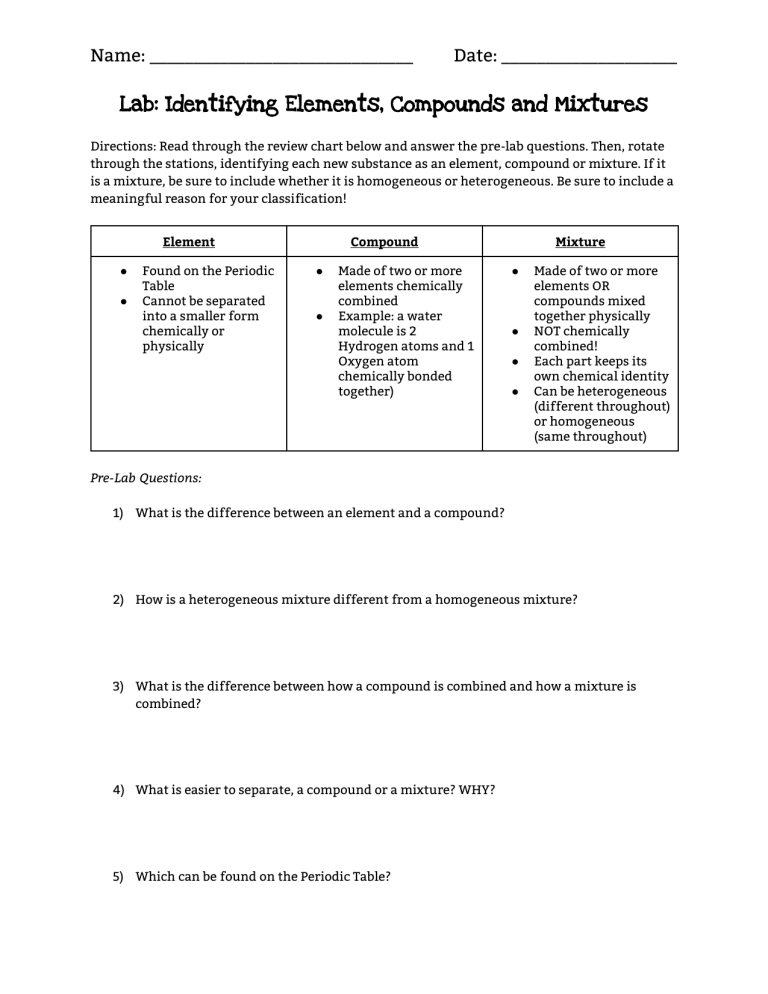
Name: ______________________________ Date: ____________________ Lab: Identifying Elements, Compounds and Mixtures Directions: Read through the review chart below and answer the pre-lab questions. Then, rotate through the stations, identifying each new substance as an element, compound or mixture. If it is a mixture, be sure to include whether it is homogeneous or heterogeneous. Be sure to include a meaningful reason for your classification! Element ● ● Found on the Periodic Table Cannot be separated into a smaller form chemically or physically Compound ● ● Made of two or more elements chemically combined Example: a water molecule is 2 Hydrogen atoms and 1 Oxygen atom chemically bonded together) Mixture ● ● ● ● Made of two or more elements OR compounds mixed together physically NOT chemically combined! Each part keeps its own chemical identity Can be heterogeneous (different throughout) or homogeneous (same throughout) Pre-Lab Questions: 1) What is the difference between an element and a compound? 2) How is a heterogeneous mixture different from a homogeneous mixture? 3) What is the difference between how a compound is combined and how a mixture is combined? 4) What is easier to separate, a compound or a mixture? WHY? 5) Which can be found on the Periodic Table? Name: ______________________________ Station Number/Name of Substance: 1.___________________ 2.___________________ 3.___________________ 4.___________________ 5.___________________ 6____________________ 7.___________________ 8.___________________ 9.___________________ 10.__________________ Classification: Element, Compound or Mixture? Date: ____________________ What Type of Mixture? (Heterogeneous or Homogeneous) How Do You Know? Name: ______________________________ Date: ____________________ KEY: 1) Oil and Water - mixture/hetero 2) Copper Wire - element 3) Chalk (CaCO3) - compound 4) Rocks and Sand - mixture/hetero 5) Water - compound 6) Koolaid (or Gatorade) - mixture/homogeneous 7) Aluminum Foil - element 8) Baking Soda (NaHCO3) - compound 9) Salt Water - mixture/homogeneous 10) Salt (NaCl) - compound Name: ______________________________ Date: ____________________ Station Cards: Station 1: Oil and Water Station 2: Copper Wire Name: ______________________________ Date: ____________________ Station 3: Chalk (CaCO3) Station 4: Rocks and Sand Name: ______________________________ Date: ____________________ Station 5: Water (H2O) Station 6: Koolaid Name: ______________________________ Date: ____________________ Station 7: Aluminum Foil Station 1: Baking Soda (NaHCO3) Name: ______________________________ Date: ____________________ Station 9: Salt Water Station 10: Salt (NaCl)




