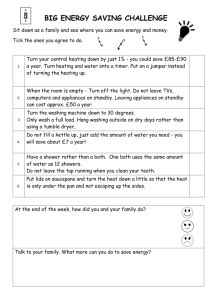
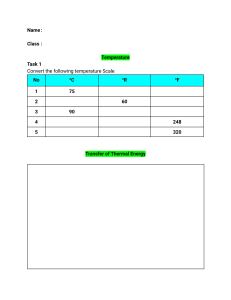
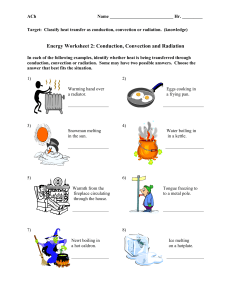
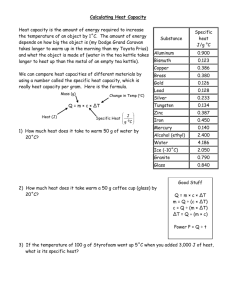
MEEN10050 Water Heating Expt SAMPLE EXPERIMENTAL READINGS 25 03 2020
advertisement

MEEN 10050 Experiment #1: Water Heating – SAMPLE EXPERIMENTAL DATA Experimental Readings: Experiment 1, Part A (Heating of Liquid in an Electric Kettle) m1 = Mass of empty kettle (kg) 1.0 m2 = Mass of kettle after water addition (kg) 1.99 Average Electric Power Consumption during water heating (kW) 2.2 o TK-initial = Initial Water Temperature ( C) 21 TK-final = Final Water Temperature (oC) 100 Time required to achieve boiling (seconds) 196 Range of external kettle temperatures measured using handheld non-contact thermometer (oC) 25 - 76 m4 = Mass of kettle after boiling (kg) 1.98 m5 = (m4 - m3) = Mass of water evaporated during boiling (kg) 0.016 Computed Results: Experiment 1, Part A (Heating of Liquid Water in an Electric Kettle) m3 = (m2 - m1) = Mass of water added to empty kettle (kg) Total electrical energy input during water heating (kJ) No. “Units” of Electricity Used during Water Heating Note: 1 “unit” of electricity = 1 kW-hr = 3600 kJ = 3.6 MJ Approximate Cost of Heating 1 kg water to boiling temperature (Domestic Electricity costs) € Cost of 1 Unit of Electricity (Domestic Use) supplied by ESB = approx. €0.25 THEORETICAL (Sensible) Energy Input Needed to Increase the Temperature of Liquid Water from TK-initial to TK-final (kJ) (Equation 2) Ratio of MEASURED / THEORETICAL Energy Inputs Difference between Measured and Theoretical Energy Inputs (kJ) Difference between Measured and Theoretical RATES of Energy Input (watts) Experimental Readings: Experiment 1, Part B (Heating of Liquid in a Microwave Oven) m9 = Mass of empty kettle (kg) 1.0 m10 = Mass of kettle after water addition (kg) 1.501 Average Electric Power Consumption during water heating (kW) 1.2 TM-initial = Initial Water Temperature (oC) 21 o TM-final = Final Water Temperature ( C) 99 Time duration of microwave heating (seconds) 179 m11 = Mass of kettle after boiling (kg) 1.496 MEEN10050 Experiment 1 Water Heating – Data Recording Sheet (measured & computed) 1 Computed Results: Experiment 1, Part B (Heating of Liquid Water in a Microwave Oven) m3 = (m2 - m1) = Mass of water added to empty water jug (kg) Total electrical energy input during microwave heating (kJ) THEORETICAL Energy Input Needed to Increase the Temperature of Liquid Water from TMinitial to TM-final (kJ) (Equation 2) Ratio of MEASURED / THEORETICAL Energy Inputs Difference between Measured and Theoretical Energy Inputs (kJ) Average Difference between Measured and Theoretical RATES of Energy Input (watts) m12 = (m10 - m11) = Mass of water evaporated during microwave heating (kg) Experiment 1, Part C: Continuous Water Boiling in an Electric Kettle Experimental Readings: Experiment 1, Part C (Continuous Water Boiling in an Electric Kettle) 1.98 m6 = Mass of kettle + water after re-boiling (kg) Indicated Water Temperature during Continuous Boiling (oC) 100 o External kettle temperature measured using handheld non-contact thermometer ( C) 35 - 87 Time period during which continuous boiling occurred tb (seconds) 60 m7 = Mass of kettle + water after period of continuous boiling (kg) 1.90 Average Electric Power Consumption during continuous boiling (kW) 2.2 Computed Results: Experiment 1, Part C (Continuous Water Boiling in an Electric Kettle) m8 = (m6 - m7) = Mass of water evaporated during continuous boiling (kg) Rate of liquid mass loss due to boiling m& evap. = (m8/tb) (kg.s-1) THEORETICAL Rate of (latent) energy input needed to boil m8 kg of water at 100oC in time tb (kW) (Equation 4) Ratio of MEASURED / THEORETICAL rates of Energy Inputs Note: This document is intended for use by students in the laboratory for convenient recording of data and tabulation of computed parameters during the experiment. It should not be submitted as part of the Laboratory Report. Please complete the Report Template file separately for final submission. MEEN10050 Experiment 1 Water Heating – Data Recording Sheet (measured & computed) 2