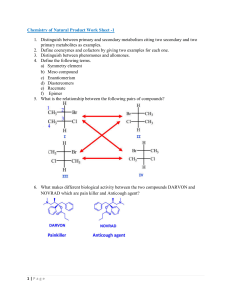
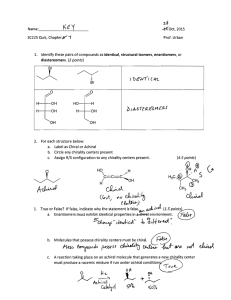
Module 5 ISOMERISM AND CHIRALITY //kdo Isomerism and Chirality Topic 5.1 CONSTITUTIONAL ISOMERISM 2 Isomerism and Chirality Isomerism • Isomers – Are compounds having the same numbers and kinds of atoms but differ in the way the atoms are arranged. 3 Isomerism and Chirality Types of Isomers 4 Isomerism and Chirality Constitutional (Structural) Isomers “Different CONNECTIVITY” • Skeletal - Different carbon skeletons • Functional - Different functional groups • Positional - Different position of functional groups 5 Isomerism and Chirality Constitutional (Structural) Isomers • Skeletal - Different carbon skeletons butane 2-methylpropane 6 Isomerism and Chirality Constitutional (Structural) Isomers • Functional - Different functional groups 7 Isomerism and Chirality Constitutional (Structural) Isomers • Positional - Different position of functional groups 8 Isomerism and Chirality Constitutional (Structural) Isomers 9 Isomerism and Chirality Activity 1 • Lay a white bond paper on a flat surface. • Using 5 toothpicks, construct a linestructure of HEXANE, as shown below. 10 Isomerism and Chirality Activity 1 • From this set-up, move the sticks to make a different structure. – No cyclic, multiple bonds or addition/reduction of sticks • Name it and list down on a separate bond paper. • Repeat until you complete five (5) different isomers of hexane (including hexane itself). 11 Isomerism and Chirality Five (5) Isomers of Hexane 12 Isomerism and Chirality Topic 5.2 CONFORMATIONAL ISOMERISM: DRAWING NEWMAN PROJECTIONS 13 Isomerism and Chirality STEREOISOMERS • Stereochemistry – the branch of chemistry concerned with the three-dimensional structures of molecules. • Stereoisomers – Same molecular formula and constitution but different spatial (3D) arrangement of atoms – SAME CONNECTIVITY 14 Isomerism and Chirality Conformational Isomers • Conformers/ Rotamers – Different arrangement due to rotation around a SINGLE BOND 15 Isomerism and Chirality Activity 2 • Construct a ball and stick 3D model of ETHANE using molding clay (atoms) and toothpicks (bonds). 16 Isomerism and Chirality Activity 2 • Take a snapshot after 90o of rotation (looking directly in front of a C-C bond end on). • On a bond paper, sketch a drawing of ETHANE using skeletal structures (label the H-atoms). 17 Isomerism and Chirality Representations of Ethane 18 Isomerism and Chirality Wedge and Dash Diagrams: 19 Isomerism and Chirality Wedge and Dash Diagrams: 20 Isomerism and Chirality Representations of Ethane 21 Isomerism and Chirality Representations of Ethane 22 Isomerism and Chirality Exercise: • Draw the Newman projection of the given compound, as viewed from the angle indicated. 23 Isomerism and Chirality FRONT: Cl Br CH3 24 Isomerism and Chirality Back: CH3 Br Cl 25 Isomerism and Chirality NEWMAN PROJECTION: Br CH3 Br Cl Cl CH3 26 Isomerism and Chirality Exercise: • Draw the Newman projection of the given compound, as viewed from the angle indicated. 27 Isomerism and Chirality FRONT: Cl H CH3 28 Isomerism and Chirality Back: CH3 Br H 29 Isomerism and Chirality NEWMAN PROJECTION: H CH3 Br Cl H CH3 30 Isomerism and Chirality Conformations of Ethane • Rotate the model and take snapshots following this diagram. 31 Isomerism and Chirality Conformations of Ethane • Staggered (99%): – The lowest-energy, most stable conformation – all six C–H bonds are as far away from one another as possible • Eclipsed (1%): – The highest-energy, least stable TORSIONAL ANGLE conformation – six C–H bonds are as close as possible 32 Isomerism and Chirality Conformations of Butane STERIC STRAIN – REPULSION AS A RESULT OF TRYING TO OCCUPY THE SAME SPACE 33 Isomerism and Chirality Topic 5.3 GEOMETRIC ISOMERISM: CIS-TRANS ISOMERS AND THE E-Z CONVENTION 34 Isomerism and Chirality Activity • Construct a ball and stick 3D model of but-2-ene using molding clay (atoms) and toothpicks (bonds). 35 Isomerism and Chirality Geometric (Cis-Trans) Isomers • Lack of rotation in C=C bonds • For disubstituted alkenes • Example: but-2-ene cis-but-2-ene trans-but-2-ene 36 Isomerism and Chirality Exercise: • Name the structure and assign the configuration of double bond as either cis- or trans-. 37 Isomerism and Chirality Exercise: • Name the structure and assign the configuration of double bond as either cis- or trans-. trans-1,2-dibromoethene 38 Isomerism and Chirality Exercise: • Name the structure and assign the configuration of double bond as either cis- or trans-. 39 Isomerism and Chirality Exercise: • Name the structure and assign the configuration of double bond as either cis- or trans-. cis-1,2-dibromoethene 40 Isomerism and Chirality Geometric (Cis-Trans) Isomers • When two identical groups are connected to the same position, there cannot be cis-trans isomerism. 41 Isomerism and Chirality Example: NOT ISOMERIC 1,1-dichloroprop-1-ene 42 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. 43 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. A trans-isomer 44 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. 45 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. A cis-isomer 46 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. 47 Isomerism and Chirality Exercise: • Classify if the following structure is a cis-, trans-, or neither an isomer. Not isomeric 48 Isomerism and Chirality Geometric (Cis-Trans) Isomers • Which of the following is less stable? cis-but-2-ene or trans-but-2-ene? cis-but-2-ene trans-but-2-ene 49 Isomerism and Chirality Geometric (Cis-Trans) Isomers • Which of the following is less stable? cis-but-2-ene or trans-but-2-ene? 50 Isomerism and Chirality Geometric (Cis-Trans) Isomers • Although the interconversion of cis and trans alkene isomers doesn’t occur spontaneously, it can be brought about by treating the alkene with a strong acid catalyst. 51 Isomerism and Chirality Sequence Rules: The E,Z Designation • With trisubstituted and tetrasubstituted double bonds, however, a more general method is needed for describing double-bond geometry. • According to the E, Z system of nomenclature, a set of sequence rules is used to assign priorities to the substituent groups on the double-bond carbons. • Cahn–Ingold–Prelog rules 52 Isomerism and Chirality Sequence Rules: The E,Z Designation • If the higher-priority groups on each carbon are on opposite sides of the double bond, the alkene is designated E, for the German entgegen, meaning “opposite.” 53 Isomerism and Chirality Sequence Rules: The E,Z Designation • If the higher-priority groups are on the same side, the alkene is designated Z, for the German zusammen, meaning “together.” – “ze zame zide” 54 Isomerism and Chirality Sequence Rules: The E,Z Designation • RULE 1 Taking the double-bond carbons separately, look at the atoms directly attached to each carbon and rank them according to atomic number. – An atom with a higher atomic number is higher in priority than an atom with a lower atomic number. 55 Isomerism and Chirality Sequence Rules: The E,Z Designation • RULE 2 If a decision can’t be reached by ranking the first atoms in the substituents, look at the second, third, or fourth atoms away from the double-bond carbons until the first difference is found. 56 Isomerism and Chirality Sequence Rules: The E,Z Designation 57 Isomerism and Chirality Sequence Rules: The E,Z Designation RULE 3 Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. 58 Isomerism and Chirality Exercise: • Assign the configuration of double bond as either E- or Z-. 59 Isomerism and Chirality Exercise: (Z)- 1-bromo-2-chloro-2-fluoro-1-iodoethene Higher priority Higher priority 60 Isomerism and Chirality Exercise: • Assign the configuration of double bond as either E- or Z-. 61 Isomerism and Chirality Exercise: • Assign the configuration of double bond as either E- or Z-. Higher Higher priority priority O C H C H C Z-isomer 62 Isomerism and Chirality Topic 5.4 STEREOISOMERISM: CHIRALITY AND SYMMETRY 63 Isomerism and Chirality Stereochemistry • Chemistry concerned with 3D structures • Focus on chirality (ky-ral-i-tee), or handedness (Greek cheir, meaning “hand”) 64 Isomerism and Chirality Chirality • Any object can be viewed in a mirror, revealing its mirror image. 65 Isomerism and Chirality Chirality • SUPERIMPOSABLE – The mirror image is identical to the actual object. 66 Isomerism and Chirality Chirality • NONSUPERIMPOSABLE – The object and its mirror image are different. 67 Isomerism and Chirality CHIRALITY • Finding Handedness in Molecules – The presence of a carbon atom bonded to four different groups stereocenter, or chirality center 68 Isomerism and Chirality Stereocenter, or chirality center 69 Isomerism and Chirality CHIRALITY AND SYMMETRY • Plane of symmetry • a plane that cuts through the middle of a molecule or other object so that one half of the object is a mirror image of the other half. • A molecule is not chiral (achiral) if it contains a plane of symmetry. 70 Isomerism and Chirality Which pair of figures has plane of symmetry? 71 Isomerism and Chirality Optical Activity • Ability to rotate a beam of plane-polarized light • Polarimeter – Measures the amount of rotation • Some optically active molecules rotate planepolarized light – to the right (clockwise) Dextrorotatory (+) – to the left (counterclockwise) Levorotatory (-) 72 Isomerism and Chirality Pasteur’s Discovery of Enantiomers 73 Isomerism and Chirality Racemetes • A.k.a. racemic (rah-see-mic) mixtures • 50:50 mixture of enantiomers 74 Isomerism and Chirality Chirality Centers and Stereoisomers Maximum number of stereoisomers = 2n – Where n = number of chirality centers 75 Isomerism and Chirality Enantiomers • Stereoisomers that are nonsuperimposable mirror image 76 Isomerism and Chirality Diastereomers • Stereoisomers that are NOT mirror images of each other Meso Compounds • Compounds that are achiral, yet contain stereocenters 77 Isomerism and Chirality 78 Isomerism and Chirality Topic 5.5 STEREOGENIC CENTERS AND THE R-S CONVENTION 79 Isomerism and Chirality Chirality • Objects that are not superimposable on their mirror images are called chiral objects. • CHIRALITY CENTER – The most common source of molecular chirality is the presence of a carbon atom bearing four different groups. – Chiral center, stereocenter, stereogenic center, asymmetric center, CHIRAL CARBON 80 Isomerism and Chirality Chirality • CHIRALITY CENTER 81 Isomerism and Chirality 2-butanol 82 Isomerism and Chirality Chirality Centers 83 Isomerism and Chirality Exercise: • Propoxyphene, sold under the trade name Darvon, is an analgesic (painkiller) and antitussive (cough suppressant). Identify all chirality centers in propoxyphene: 84 Isomerism and Chirality 85 Isomerism and Chirality 86 Isomerism and Chirality Answer: 87 Isomerism and Chirality Exercise: Identify the chiral carbons. 88 Isomerism and Chirality Exercise: Identify the chiral carbons. 89 Isomerism and Chirality Enantiomers • When a compound is chiral, it will have one nonsuperimposable mirror image, called its enantiomer (from the Greek word meaning “opposite”) 90 Isomerism and Chirality Designating Configuration Using the Cahn-Ingold-Prelog System RULE 1 Look at the four atoms directly attached to the stereocenter, and assign priorities in order of decreasing atomic number. The atom with the highest atomic number is ranked first; the atom with the lowest atomic number is ranked fourth. 91 Isomerism and Chirality Designating Configuration Using the Cahn-Ingold-Prelog System RULE 2 If a decision can’t be reached by ranking the first atoms in the substituents, look at the second, third, or fourth atoms outward until the first difference is found. RULE 3 Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. 92 Isomerism and Chirality 1 4 2 3 93 Isomerism and Chirality Designating Configuration Using the Cahn-Ingold-Prelog System Rule 4 Orient the molecule so that the group of lowest priority (4) is pointing directly back, away from us. We then look at the three remaining substituents, which now appear to radiate toward us like the spokes on a steering wheel (Figure 6.7). 94 Isomerism and Chirality FRONT: 1 Cl CH3 H2C 2 CH3 3 95 Isomerism and Chirality Designating Configuration Using the Cahn-Ingold-Prelog System Priority substituent (1 2 3) is clockwise, R configuration (Latin rectus, meaning “right”). If an arrow from 1 2 3 is counterclockwise, S configuration (Latin sinister, meaning “left”). 96 Isomerism and Chirality FRONT: 1 Cl CH3 H2C CH3 2 3 97 Isomerism and Chirality (S)-2-chlorobutane 98 Isomerism and Chirality Exercise: Identify whether the structure exhibits S- or R- configuration. 99 Isomerism and Chirality Not isomeric 100 Isomerism and Chirality 101 Isomerism and Chirality 102 Isomerism and Chirality Higher priority 103 Isomerism and Chirality 104 Isomerism and Chirality 105 Isomerism and Chirality 106 Isomerism and Chirality Higher priority 107 Isomerism and Chirality Higher priority Higher priority E-isomer 108 Isomerism and Chirality 109 Isomerism and Chirality Chirality in Nature Just as different stereoisomeric forms of a chiral molecule have different physical properties, they usually have different biological properties as well. For example, the (+) enantiomer of limonene has the odor of oranges, but the (−) enantiomer has a piney scent. 110 Isomerism and Chirality Chirality in Nature 111 Isomerism and Chirality Chirality in Nature • Fluoxetine, a commonly prescribed medication sold under the trade name Prozac. • Racemic fluoxetine is an extraordinarily effective antidepressant, but it has no activity against migraine. • The pure S enantiomer, however, works remarkably well in preventing migraine and is now undergoing clinical evaluation. 112 Isomerism and Chirality Chirality in Nature • Why do different stereoisomers have different biological properties? • To exert its biological action, a chiral molecule must fit into a chiral receptor at a target site 113 Isomerism and Chirality - End of Presentation - For questions, you can post them on the discussion page in CANVAS. 114