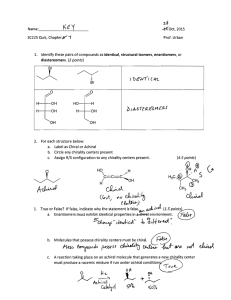
Chemistry of Natural Product Work Sheet -1 1. Distinguish between primary and secondary metabolism citing two secondary and two primary metabolites as examples. 2. Define coenzymes and cofactors by giving two examples for each one. 3. Distinguish between pheromones and allomones. 4. Define the following terms. a) Symmetry element b) Meso compound c) Enantiomerism d) Diastereomers e) Racemate f) Epimer 5. What is the relationship between the following pairs of compounds? 6. What makes different biological activity between the two compounds DARVON and NOVRAD which are pain killer and Anticough agent? 1|Page 7. Place asterisks at all the chirality centers in each molecule below. a) b) c) 8. What is meant by the term absolute configuration and how is it specified? 9. Assign R, S configurations to each indicated chirality center in the molecules below. a) b) 2|Page c) d) 10. Consider that (S)- bromobutane has a specific rotation of +23.10 and(R)-bromobutane has specific rotation of -23.10 a) Determine the optical purity of a racemic mixture. b) Which isomer is dominant and what is the optical purity of a mixture, of (R)- and (S)- bromobutane,whose specific rotation was found to be -9.20 3|Page Chemistry of Natural Product Assignment-1 1. Why is natural product and secondary metabolism so widely variable in the plant kingdom? 2. Why do plants produce secondary metabolites? 3. Lemon and Orange contains Limonene. What difference they have from the angle of stereo chemistry? Describe by drawing their structures? 4. Assign R, S configurations to each indicated chirality center in the molecules below. a) b) 4|Page 5. Explain briefly (in one or two short sentences) the meaning of the following basic stereochemical terms. a) chirality b) Constitution c) Configuration d) Conformation e) Stereoisomers 6. Menthol is a member of the terpene family of natural products. It exists in a (1R, 2S, 5R) form and a (1S, 2R, 5S) form. Are these two compounds enantiomers or diastereomers? 7. Define the following terms. a) Stereoselectivity b) stereospecific 5|Page