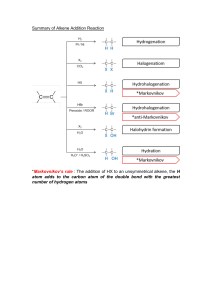
CHEM - THINGS TO RECITE isotopes - atoms of an element which have the same number of protons but different number of neutron allotropes - different forms of the same element Fossil Fuel - fuels form from dead remains of living(coal : land // marine : petroleum and natural gas) organism that lives millions of years ago Cracking - process where larger molecules are break down into smaller molecules by heat with the help of a catalyst Structural Isomers - same molecular formula different structural formula (chain // position // functional group) Chain Isomers - same functional groups , different carbon skeleton Position Isomers - same functional group , different position of them Functional Group Isomers - different functional group Stereoisomerism - atoms linked in the same way but differ in spatial arrangement of atoms Enantiomers - cause same extent of rotation of plane of plane-polarized light in opposite direction , using a polarimeter Disadvantages of track progress with titration - difficult to monitor change in concentration continuously - cannot be used to study fast reaction as titrimetric analysis takes time Filter - select light then coloured species absorb the most Catalyst - substance that changes the rate of reaction while itself remains chemically unchanged Avogadro’s law - equal volume of all gasses contain equal number of gas molecules Characteristics of Chemical Equilibrium 1. dynamic in nature , the forward and backward reaction do not stop when the equilibrium is reached 2. forward reaction rate equal to backward reaction rate and is not zero 3. exists only in a close system 4. can be reached from either forward or backward direction Periodicity - repeating pattern of physical and chemical properties shown by different periods Properties of Transitional Metals 1. coloured compounds : iron (II) chloride // iron (III) chloride 2. existence of more than one oxidation state in compound : +2 // +3 3. catalytic properties of metal and compound : haber process // Decomposition of hydrogen peroxide Hess Law - the total enthalpy change of a chemical reaction is independent of the route by which the reaction takes place Why enthalpy change cannot be found directly 1. formation of side product (carbon dioxide from formation of carbon monoxide) 2. reaction rate to slow (rust) 3. too dangerous to be performed in lab (potassium to acid) 4. extent of reaction cannot be controlled 5. direct combustion is vigorous Enthalpy change - heat change at constant pressure Standard Enthalpy change of combustion - the enthalpy change of complete combustion of one mole of the substance in oxygen under standard conditions Below, you will find an overview (non-exhaustive!) of all reactions in the HKDSE Chemistry syllabus, DO NOT rely only on the following list, it serves only to give you a general sense and final reminders. Metal extraction 1. Metal ores → metal + O2 (direct heating) 2. Metal ores + C → metal + CO2 (carbon reduction) Metal reactions 1. Metal + O2 → metal oxide 2. Metal + water → metal hydroxide + H2 3. Metal + steam → metal oxide + H2 4. Metal + acid → salt + H2 (not water) 5. 4Fe(s) + 3O₂(g) + 2nH₂O(l) → 2Fe₂O₃ . nH₂O(s) (rusting) Acid reactions 1. Dilute acid + metal → salt + H2 2. Acid + base → salt + water (neutralisation) 3. Acid + CO3 2- → salt + water + CO2 4. Acid + HCO3- → salt + water + CO2 5. Acid + SO3 2- → salt + water + SO2 6. Acid + HSO3- → salt + water + SO2 Base reactions 1. Base + ammonium salt → salt + water + NH3 2. Base + acid → salt + water (neutralisation) Alkanes 1. Fuel + O2 → CO2 + H2O (complete combustion) a. Toxic CO is produced under incomplete combustion 2. Alkane + Cl2/Br2 → haloalkane (substitution) a. To differentiate alkane vs alkene i. Alkane: bromine under UV light ii. Alkene: bromine in the dark 3. Large alkane → small alkane + small alkene (cracking) a. Heat in absence of oxygen under high pressure / catalyst Alkenes 1. Alkene + Cl2/Br2 → haloalkane (addition) 2. Alkene → alcohol (addition) a. With cold alkaline dilute KMnO4 i. Just recite it, I don't know why its cold alkaline too =) 3. Alkene → polymer (addition polymerisation) Redox You must refer to your own notes for redox and you must be able to 1. Recite all common OA RA by heart 2. Know their colour changes 3. Know how to find ON 4. Know how to write and balance redox equation 5. Recite the two chemical cells by heart Section below is dedicated to reactions (conversions) of carbon compounds Alkane 1. Alkane + Cl2/Br2 → haloalkane Alkene 1. Alkene + H2 → Alkane a. Pt / Ni with heat 2. Alkene + Cl2/Br2 → haloalkane 3. Alkene + HX → haloalkane 4. Alkene → alkanol (diol) a. Cold alkaline KMnO4 Haloalkane RX 1. RX + OH- → ROH (alcohol) Alcohol ROH 1. ROH + HX / PX3 → haloalkane 2. ROH → alkene (dehydration) a. Conc H2SO4 / Al2O3 with heat 3. Primary ROH → aldehyde (oxidation) a. Acidified Cr2O7 / MnO44. Secondary ROH → ketone (oxidation) a. Acidified Cr2O7 / MnO4- Aldehyde 1. Aldehyde → RCOOH (carboxylic acid) need to lerant spelling! a. Acidified Cr2O7 / MnO4-, heat under reflux 2. Aldehyde / Ketone → alcohol a. 1)LiAlH4 in dry ether 2) H+ / NaBH4 RCOOH 1. RCOOH + ROH → ester + water (esterification) a. Conc H2SO4, heat 2. RCOOH → primary ROH a. 1)LiAlH4 in dry ether 2) H+ / NaBH4 3. RCOOH + NH3 → amide a. Heat dont forget about this reaction! Ester 1. Ester → RCOOH + ROH (acid hydrolysis) a. H+, heat 2. Ester → R-COO- + R-OH (alkaline hydrolysis) a. OH-, heat Amide 1. Amide → RCOOH + NH4+ (acid hydrolysis) a. H+, heat 2. Amide → RCOO- + NH3 (alkaline hydrolysis) a. OH-, heat Refers to the conversion diaphragm for details in 24 hours chemistry book Questions types / topics that MUST appear in your HKDSE exam 1. Reactions related to metals 2. Reactions related to acid and base 3. Chemical cell & electrolysis setup 4. Mole calculation 5. Solubility 6. Titration procedures and calculation 7. Rate of change & equilibrium 8. Fossil fuels