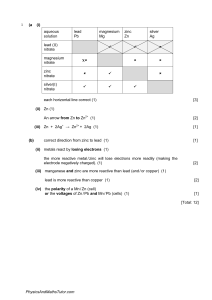
Name :
Date:
Stoichiometry Practice #1
Directions: Write and balance the chemical equations and then do stoichiometry to calculate
the answer(s) on a separate sheet of paper.
•
You must show all work to receive credit!
•
•
Label units and substance all the way through.
Answers should be in proper scientific notation if needed, decimals rounded to the
hundredths place, and labeled with units and substance.
1. Copper (II) chloride and silver nitrate react to form copper (II) nitrate and silver chloride.
d:
_\ CuCl2 + 2=_ AgN03
Gi,en: 5.2 g AgNO,
Find: particles AgCI
_( Cu(N03)2 +~Ag51
1-2_Ai,Y~ - /.q/1':Jf!o'(j
,"1 )((V:,
,r ArC/
117
I· i
70 - ~
2
V 16 -,__ fq,t ,c~') A'.51
J-t--'-'--"-'--~
2. Iron metal reacts wit bromine gasto form iron (1) 6romide: - - - - - ~ - - - -Write the balanced chemical equatio2Given: 7.5 g iron
·
Fe
\
f--_l_
n(7F(
3. Pentane reacts with oxygen to form carbonp.oxide and water.
_\_ CsH12 +
Given: 92 L CsH12
Find: grams of CO2
+
2
--
(3
I),,-- 1. _ff' r'
7- ',,i j {,../e F('J ( ! rrJ~~,- r(! ¾?
-
Find: grams of iron (I) bromide
1
-{J.02
_G_ H20
2
l!~1r- Ff!
. 0)3 /-r
1. F
I )
tl
[!"1:1r,~, II ~-.,t<l ~!
j ~ t . j - -!.... ;..:At f
,.,ti~ ...:"-~--'"'.:.~:~
~• V
2,.. ~ ~"'C. l ""'
;1Jll, · ,
C,1vc n l
-
I
r
I
,i,-, 10'' par: cles I;( D,
I
J
l
I
'1