Elements, Compounds, Mixtures Worksheet
advertisement

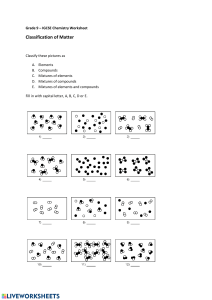
Key Learning Elements, Compounds and Mixtures Differences between elements, compounds and mixtures can be described by using a particle model (VCSSU097) modelling the arrangement of particles in elements and compounds recognising that elements and simple compounds can be represented by symbols and formulas explaining why elements and compounds can be represented by chemical formulas while mixtures cannot Be able to define an atom and an element and understand that they make up matter. 1.All matter is made up of tiny particles called atoms 2.The particles of matter are always moving 3.The particles have spaces between them 4.Adding heat to matter makes the particles move faster Recognize that elements and simple compounds can be represented by symbols and formulas Atoms bond together to make molecules and elements Recognize that elements form a molecule or compound. Be able to describe the difference between a molecule and a compound Molecules contain only one type of element Compounds contain more than one type of element The Periodic Table Be able to explain the information the Periodic Table gives us Describe how the trends and patterns in the periodic table relate to reactivity and properties of the elements listed Be able to identify and locate elements on the periodic table Be able to list the symbols for the first twenty elements Describe what a group and a period is in the table