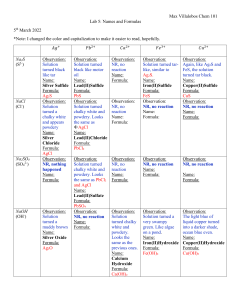
Chem 101 Lab 5: Names and Formulas 5th March 2022 *Note: I changed the color and capitalization to make it easier to read, hopefully. Na2S (S2-) NaCl (Cl-) Na2SO4 (SO42-) NaOH (OH-) 𝐴𝑔+ 𝑃𝑏 2+ Observation: Solution turned black like tar Name: Silver Sulfide Formula: Ag₂S Observation: Solution turned a chalky white and appears powdery Name: Silver Chloride Formula: AgCl Observation: NR, nothing happened Name: Formula: Observation: Solution turned black like motor oil Name: Lead(II)Sulfide Formula: PbS Observation: Solution turned chalky white and powdery. Looks the same as AgCl Name: Lead(II)Chloride Formula: PbCl₂ Observation: NR, no reaction Name: Formula: Observation: Solution turned chalky white and powdery. Looks the same as PbCl₂ and AgCl Name: Lead(II)Sulfate Formula: PbSO₄ Observation: NR, no reaction Name: Formula: Observation: Solution turned a muddy brown Name: Silver Oxide Formula: Ag₂O 𝐶𝑎2+ 𝐹𝑒 3+ 𝐶𝑢2+ Observation: Solution turned tarlike, similar to Ag₂S. Name: Iron(II)Sulfide Formula: FeS Observation: NR, no reaction Name: Formula: Observation: Again, like Ag₂S and FeS, the solution turned tar black. Name: Copper(II)Sulfide Formula: CuS Observation: NR, no reaction Name: Formula: Observation: NR, no reaction Name: Formula: Observation: NR, no reaction Name: Formula: Observation: NR, no reaction Observation: Solution turned chalky white and powdery. Looks the same as the previous ones. Name: Calcium Hydroxide Formula: Ca(OH)₂ Observation: Solution turned a very swampy green. Like algae on a pond. Name: Iron(II)Hydroxide Formula: Fe(OH)₂ Observation: The light blue of liquid copper turned into a darker shade, ocean blue even. Name: Copper(II)Hydroxide Formula: Cu(OH)₂ Observation: NR, no reaction Name: Formula: Name: Formula: Na2CO3 (CO32-) Observation: Solution turned a very light brown with a gloss to it Name: Silver Carbonate Formula: Ag₂CO₃ Observation: Solution turned chalky white and powdery. Looks the same as the previous ones. Looks the same as PbCl₂, AgCl, and PbSO₄ Name: Lead(II)Carbonate Formula: PbCO₃ Observation: Solution turned chalky white and powdery. Looks the same as the previous ones. (Very interesting.) Name: Calcium Carbonate Formula: CaCO₃ Chem 101 Observation: Observation: Solution turned a The light blue of dark, dirty green. liquid copper stayed Looks murky. relatively the same in Name: color shade, however, Iron(II)Carbonate it appears chalkier Formula: with a white tinge to FeCO₃ it. Name: Copper(II)Carbonate Formula: CuCO₃ 1. Did you notice any patterns? Explain the patterns you noticed. I did notice patterns. A few reactions yielded a solution that was tar-like in appearance (Row 1). My guess is because of the sulfur turning it black mid reaction. Furthermore, one reappearing pattern was the powdery-white effect Na2CO3 seemed to cause (Last row). The solution would look rather thick in consistency and chalky. However, NaOH and Na2SO4 both had this effect in some other solutions. My hypothesis is that since Na is present in each reaction, it is making the produced solution chalky. I am not sure why that is however.