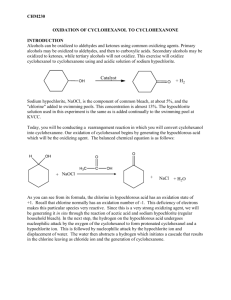
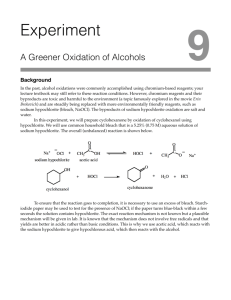
lOMoARcPSD|12644504 Oxidation of Cyclohexanol Lab Report Organic Chem 1 (Lab) (Hunter College CUNY) StuDocu is not sponsored or endorsed by any college or university Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 Chem 223 Lab Report #10 Oxidation of Cyclohexanol to Cyclohexanone With Sodium Hypochlorite Solution + Acetic Acid Objective Oxidize secondary alcohol (cyclohexanol) to a ketone (cyclohexanone) with a sodium hypochlorite solution plus acetic acid. Physical Compounds Name Structure Of Molecule MP (℃) BP (℃) Density (g/cm3) Solubility In Water At 25 ℃ Hazards Sodium Hypochlorite 18 101 1.11 293 g/L at 20℃ -Exposure can cause headache, dizziness, nausea, and vomiting Cyclohexanol 25.93 161.8 962 kg/m^3 42 g/L -Contact can irritate and burn the skin and eyes -Breathing can irritate the nose and throat -High exposure can cause headache, nausea, vomiting, dizziness, and passing out Cyclohexanone -31 155.6 948 kg/m^3 25 g/L -Cracking of the skin with redness and watery blisters -Inhaling can irritate the nose and throat -Exposure can cause headache, dizziness, Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 lightheadedness, and passing out Sodium Thiosulphate 48.3 100 1.67 70.1 g/100 mL -May cause mild eye irritation -May cause skin irritation -Ingestion of large amounts may cause gastrointestinal irritation -May cause respiratory tract irritation Sodium Sulfate 884 1,429 2.66 445.47 g/L -Gives off irritating or toxic fumes -Dust can cause temporary asthma or eye irritation Sodium Bicarbonate 801 1,465 2.2.16 36.0 g/100 -Gastrointestinal irritation can occur if large amounts have been ingested -Mild irritation, such as redness and slight pain, may result from eye contact Acetic Acid 16.6 118 1.05 100 g/L -Severe irritant of the eyes, mucous membranes, upper respiratory tract, and skin Procedure Addition of Sodium Hypochlorite Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 ● Pour 2mL of cyclohexanol into a 250mL Erlenmeyer flask and then add 1mL of glacial acetic acid to the cyclohexanol. ● Add a magnetic stir-bar to the flask and place the flask on top of a magnetic stirrer in the fume hood. (Do not turn on heat with the stirrer) ● Pour 60mL of sodium hypochlorite solution (approx. 0.74M) into the flask in small portions over a period of about 2 mins while the solution is stirring. ● Reaction is exothermic, and will notice that the flask will become warmer as the reaction progresses. ● Continue stirring the reaction for 20 mins in the fume hood after the addition is complete. ● During that 20 min, check about every 5 min to see if oxidant is still present. To do this, place a small piece of potassium iodide-starch indicator paper on a watch glass. ● By using a glass rod, place a drop of the reaction mixture onto the indicator paper. (Make sure to dip the rod into the aqueous layer and not just the organic layer) ● If the paper continues to change to a blue-black color at the end of 20 min, proceed to the quench step. ● If the paper stays colorless when tested (oxidizing agent not present), add an additional 5mL of sodium hypochlorite solution. ● Stir the reaction for the entire 20 mins, plus an additional 5 mins if you added oxidant at the end of that 20 min. ● Test the solution for the oxidizing agent at the end of that 5 min. ● Repeating the procedure desired above. ● If the oxidizing agent is still not present add an additional 5mL of sodium hypochlorite and stir the reaction for a further 5 mins. Quench Step ● Add 1g of solid sodium thiosulphate to the reaction mixture and stir for 2 mins. ● Check for the presence of the oxidizing agent by using the iodide-starch indicator paper as described previously. (No oxidizing agent should be present at this stage) ● Check the pH of the reaction mixture by transferring a drop of the reaction mixture from the reaction to a strip of blue litmus paper on a watch glass. ● If the solution is acidic add 2g of solid sodium bicarbonate, mix well, and repeat the pH test. Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 ● If the reaction mixture is still acidic, add another 1g of sodium bicarbonate to neutralize the solution. Workup & Extraction of Product ● Add 5g of solid sodium chloride solution to the mixture. (This is done to decrease the solubility of the product (cyclohexanone) in the aqueous phase) ● Stir the solution to dissolve most of the sodium chloride. ● If it all dissolves, add more sodium chloride and stir to dissolve as before. ● Very carefully decant the liquid from the solid sodium chloride that remains undissolved into a separatory funnel. A thin, colorless, organic layer should be present on top of a cloudy aqueous layer in the separatory funnel. ● Allow the solution to remain undisturbed in the separatory funnel for about five mins. ● Open the tap on the separatory funnel and drain the bottom (aqueous) layer slowly into a small Erlenmeyer flask. ● Then drain the organic layer into a vial. (May notice a bubble of the aqueous layer in the organic layer at this stage & this is ok since the water can be easily removed.) ● To do this, place a Pasteur pipette into the solution and carefully remove the bubble of the aqueous layer at the bottom of the vial. ● May remove some of the organic layer in the pipette when this is done, but the layers will separate in the pipette. ● Using the pipette as a mini-separatory funnel, drain the bottom layer back into the Erlenmeyer flask, being careful not to transfer any of the top layer in the process. ● Place the organic (top) layer back into the vial with the rest of the product. ● Add sodium sulfate or magnesium sulfate as a drying agent. (Be careful not to use too much drying agent as this will cause loss of product) ● Allow the liquid to remain on the drying agent and occasional agitation for about 5 mins. ● The treated liquid should be clear, not cloudy ● Transfer the product from the drying agent with a clean, dry Pasteur pipette into a clean pre-weighed vial. ● Reweigh the vial to determine the yield. Spectroscopic Analysis of the Product ● Obtain an infrared spectrum of your product. Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 ● Examine the peaks in the IR spectrum and determine if cyclohexanol and/or cyclohexanone are present. ● If cyclohexanol is present, there will be an alcohol (-OH) stretch in the infrared spectrum, although water may give you a false positive for the alcohol if not dried properly. ● If cyclohexanone is present, there will be a very strong peak corresponding to the C=O stretch for the ketone group in cyclohexanone. Introduction Oxidation numbers are very important in organic chemistry, both in an academic laboratory as well as in industry. There are numerous reagents that are useful for conducting oxidations and many of the more common ones are based on chromium. Chromium compounds are often toxic and corrosive and persist in the environment. Sodium hypochlorite is a more environmentally friendly oxidizing agent and the active ingredient in commercial household bleach. Although bleach may be toxic and corrosive, it rapidly decomposes under environmental conditions and is easily tolerated in small amounts. In an acidic environment, the hypochlorite oxidation reaction is more rapid, and thus acetic acid is used to facilitate the reaction. In order to test for oxidizing agents, a very sensitive test used with potassium iodide-starch paper can check whether the oxidizing agent has been completely used in the reaction. If the solution changes the iodide-starch paper to a blue-black color, this is an indication that the solution contains an oxidizing agent. An oxidizing agent is a compound that is present in a redox reaction that receives electrons originating from a different species. However, if the color of the iodide-starch paper remains colorless, this means that there is no oxidizing agent present. Cyclohexanol is a secondary alcohol that will be oxidized to cyclohexanone, a ketone with a sodium hypochlorite solution plus acetic acid. Cyclohexanol and cyclohexanone have similar and somewhat limited solubility in water. Due to this, the cyclohexanone product and any leftover starting material will form a layer separate from the aqueous layer at the end of the reaction. Sodium chloride is added in order to decrease the solubility of cyclohexanone in water. Results Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 Figure 1: IR of reactant, cyclohexanol. Figure 2: IR of product, cyclohexanone. Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 Figure 3: IR spectrum of starting material, cyclohexanol (green) and product, cyclohexanone (red) Percent Yield: Cyclohexanone = 17.02g Weight of empty vial = 3.46g Weight of cyclohexanone = 17.02/4 = 4.25g 4.25g - 3.46g = 0.79g Organic layer weight = 0.79g 1 mole of Cyclohexanol = 100.16 g/mol Volume of Cyclohexanol = 2mL Cyclohexane = 0.96 g/mL Mass = volume X density → (2mL) X (0.96 g/mL) = 1.92g 1:1 Mole Ratio of Cyclohexanol & Cyclohexanone Cyclohexanol: Cyclohexanol Molecular Weight = 100.16 g/mol Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 Moles = (mass) / (molecular weight) = (1.92g) / (100.16 g/mol) = 0.0192 moles Cyclohexanone: Cyclohexanone molecular weight = 98.14 g/mol Mass = (mass) / (molecular weight) = (0.0192) X (98.15 g/mol) = 1.88g Percent Yield: Percent yield = (actual / theoretical) X 100 Percent yield = (0.79g X 1.88g) X 100 = 42% Discussion To conclude this experiment, it revolved around a secondary alcohol, cyclohexanol being oxidized to a ketone, cyclohexanone with the use of a sodium hypochlorite solution and acetic acid. Based on the IR spectrums of cyclohexanol and cyclohexanone it indicated that enough product was extracted which allowed the IR to be accessed clearly. When observing figure 1 which is based off of the reactant, cyclohexanol it could be noted that the expected peaks were an O-H peak between 3600-3200 cm^-1 and a C-H alkane peak between 300-2850 cm^-1 could also be found. Based on figure 2 which represents the product, cyclohexanone, expected peaks were a C=O peak between 1810-1640 cm^-1 and a C-H alkane peak between 3000-2850 cm^-1. This indicates that there was a strong absorption that occurred at 1704 cm^-1 and this is an indication of a ketone group and this specifies that cyclohexanol oxidized to cyclohexanone. Within this experiment, the percentage yield indicated that 42% of product was created. In this case there could have been an error during the experiment and it could have happened when the drying agent was added to the product. No weight was taken after adding the drying agent which meant that there was missing data and due to this it was difficult to record a theoretical yield. Another source of error could have occurred when trying to aspirate the product by using the cotton pipette. The cotton pipette was used to clean the product of sodium sulfate and there could have been a possibility that not all of the sodium sulfate was aspirated during that process, leaving some to remain when being mixed with the drying agent. A third source of error could Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com) lOMoARcPSD|12644504 have come from the other groups because based on the data, specifically the percent yield, the total amount of cyclohexanone which was 17.02 g was derived from a total of four groups. Due to this data being collective, the amount of cyclohexanone collected could have been too little because some groups could have titrated too little of the organic layer because there was not enough to begin with or it was titrated into the beaker with the aqueous layer. Conclusive Remarks Oxidation of a secondary alcohol, cyclohexanol to a ketone, cyclohexanone with a sodium hypochlorite solution plus acetic acid and using IR spectroscopy. Downloaded by Reece Garcia (hciemfzitb345x6@gmail.com)