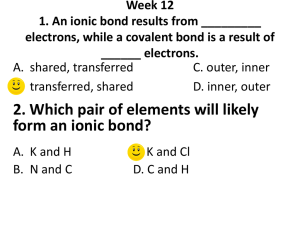
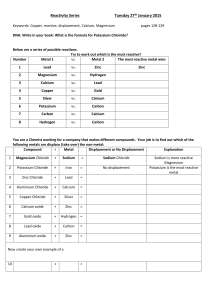
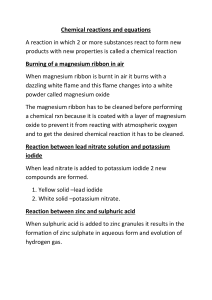
Science 10 – Chemistry Unit Exam – Review 1. Give two examples of physical changes and explain why they are physical changes. 2. What are the five indicators of a chemical change? 3. Identify the following as a chemical or physical change: a) smashing a bottle b) freezing water c) baking bread d) burning a match 4. Identify the three particles of an atom and their charges. 5. Using the atom Bromine, what is the: Symbol? Atomic number? Atomic Mass? # of Protons? # of Electrons? # of Neutrons? 6. Draw the following atoms using the Bohr model. a) lithium b) magnesium 7. What is an ion? An anion? A cation? 8. What are the differences between an ionic compound and a covalent (molecular) compound? 9. Write the name of the following compounds and indicate whether they are ionic or covalent (include roman numerals if needed) e) FeO f) CaCl2 g) P3O5 h) N5O3 e) Fe2(C2O4)3 10. Write the formulas of the following compounds and indicate whether they are ionic or covalent: a) nitrogen trioxide b) mercury (II) iodide c) calcium Sulfide d) tin (II) iodide e) tetrabromine diiodide 11. What are the seven diatomic elements and when do we use them in chemical equations? 12. Write the word, skeleton, and balanced equations for the following: a) hydrogen reacting with oxygen to produce water WE: SE: BE: b) sodium chloride breaking into sodium and chlorine WE: SE: BE: c) aluminum oxide reacting with magnesium chloride to produce aluminum chloride and magnesium oxide WE: SE: BE: 13. What is the law of the conservation of mass? Explain it in terms of the number of atoms. 14. Write the skeleton equation and then classify each of the following reactions as synthesis, decomposition, single, double replacement, or combustion reactions. a) copper + silver nitrate → silver + copper (II) nitrate b) zinc carbonate → zinc oxide + carbon dioxide c) calcium carbonate + hydrochloric acid → carbonic acid + calcium chloride d) iron + oxygen → iron (III) oxide 15. Explain how you would use your knowledge of factors that affect the rate of reaction to cook a steak as quickly as possible.