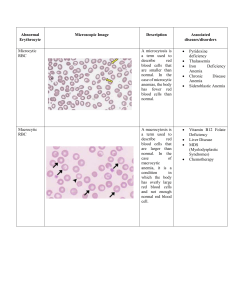
CMAP summary Tuesday, December 1, 2015 10:06 AM Anemia Monday, October 26, 2015 8:26 AM 1. What is anemia? What’s its presentation? Reduction in total circulating RBC mass Presentation (hypoxia): Pale conjunctiva and skin Weakness, fatigue, dyspnea Headache and lightheadedness Angina, especially with CAD 2. How is anemia measured? Hb, Hct and RBC count (total RBC mass difficult to measure) All of these measures are concentration dependent so have problems. Ex – in pregnancy, blood volume increases making Hb and Hct concentration low even though total amount might be same. Immediately after gunshot wound and blood loss, Hb and Hct concentration might be normal even though pt might have lost lots of blood. What is practical definition of anemia? Hb<13.5 g/dl for males and Hb<12.5 g/dl for females. (lower for females because of menstruation) What are different types of anemia? Microcytic (MCV – mean corpuscle volume <80) – small RBC Normocytic (MCV = 80-100) – normal size RBC Macrocytic (MCV > 100) – big RBC Microcytic anemia- Fe deficiency Tuesday, October 27, 2015 9:23 PM 1. What is pathophysiology of microcytic anemia? Main problem in microcytic anemia is decreased production of Hb. RBC is produced from subsequent division of erythroblast (EB). During Hb deficiency, EB divides too much. As a result, RBCs become small and microcytic anemia occurs. If erythroblast doesn’t divide enough, macrocytic anemia occurs. Think that by dividing extra, RBC surface area exposed to blood increases and it can carry more O2 – not correct idea but works for thinking Fig: microcytic anemia. Normal RBC size is equal to size of nucleus of lymphocyte. Notice that multiple RBC are smaller than that. Also notice variability in size of RBC and increased pallor in center of RBC 2. What is hemoglobin made up of? Hemoglobin = heme + globin (protein). Heme = Iron + protoprophyrin. What are etiologies of microcytic anemia (Hb deficiency)? Fe deficiency Anemia of chronic state- being unable to use Fe. In chronic inflammation, Fe is stored away in macrophage and can’t be used. Cideroblastic anemia – protoprophyrin deficiency Thalessemia – decreased production of globin Fe deficiency anemia What is epidemiology Fe deficiency anemia. Fe deficiency is the most common nutritional deficiency in the world making this the most common type of anemia (1/3rd of world is deficient in Fe) Describe digestion and storage of Fe (HY). Fe is absorbed in duodenum (HY). Protein called FERROPORTEIN plays a key role in Fe transport from lumen to enterocyte to blood. TRANSFERRIN transports iron in blood and takes it to liver and bone marrow macrophage for storage Stored intracellular iron is bound to FERRITIN There is no real way to get rid of Iron from body. So absorption by enterocytes is regulated. (some lost during skin sloughing off and menstruation) Iron is always bound to something because free Fe generates free radical by fenten reaction. What are lab measurement for Fe in body? Serum Fe – measures Fe in blood (most of it is bound to transferretin) TIBC (total iron binding capacity) – tells total transferritin in blood. Normally, 1 in every 3 transferritin in blood is bound to Fe. % saturation – % saturation of transferritin by Fe Serum ferritin – indication of how much Fe is in storage sites When ferritin↓, TIBC ↑ and vice versa(liver makes more TIBC to search for more Fe) What are some etiologies of Fe deficiency anemia? Malabsorption – Celiac Gastrectomy (HY)– Fe 2+ is absorbed easily (Fe 2 goes INTO the body). Acidic environment promotes Fe 2 conformation. When stomach is resected, due to lost acidity, more Fe will be in 3+ and Fe won’t be absorbed well. Other etiologies are based on blood loss of dietary lack Infants – breast feeding (breast milk has no Fe) Children – poor diet Adults – peptic ulcer disease (most common cause in adult males); Menorrhagia (too much bleeding during periods) or pregnancy (females). Elderly – colon polyps/carcinoma (western world); hookworm (developing world) What are stages of iron deficiency anemia? Depletion of iron storage (low serum ferretin) Depletion of serum iron Normocytic anemia (HY) – first there will be few but normal sized RBC Microcytic, hypochromic anemia (central pallor in RBC is big) Know that Normocytic anemia is followed by microcytic in Fe deficiency anemia What are clinical presentation of iron deficiency anemia? Anemia Koilonychia (spoon shaped nails) Pica (psychological drive to eat dirt – perhaps to get Fe) What are lab findings of Fe deficiency anemia? Microcytic, hypochromic anemia with ↑RDW (RDW is like standard deviation of size of RBC; larger the variation in RBC sizes, larger the RDW) – due to transition from normocytic to microcytic anemia ↓ferritin, ↑TIBC ↓serum iron, ↓%saturation ↑FEP (free erythrocyte protoporphyrin) Why is there ↑FEP in Fe deficiency anemia? As Fe is low but protoporphyrin is normal, some protoporphyrin will be unbound to Fe hence increasing the FEP. What is blood smear finding in Fe deficiency anemia? Poikilocytosis (variable shapes), anisocytosis (variable size), cigeratte shaped RBC (classic finding), tear drop RBC Microcytic anemia (note RBC smaller than lymphocyte nucleus) What is treatment of Fe deficiency anemia? Iron supplement – Ferrous sulfate Rule out any risk factors (ex – if old people, rule out colon carcinoma) What is Plummer-Vinson syndrome? Iron deficiency anemia with esophageal web and atrophic glossitis (smooth tongue due to lack of white papillae- beefy red appearance) Microcytic anemia 2 - anemia of chronic disease Tuesday, October 27, 2015 9:24 PM Anemia of chronic disease 1. What is epidemiology of anemia of chronic disease (ACD)? Most common anemia in hospitalized pt. 2. What is pathophysiology of ACD? During acute/chronic inflammation, acute phase proteins are produced. One of them is Hepcidin. It has 2 functions that results in anemia ↓Erythropoietin production Increased sequestering of Fe in macrophage. Less transfer of Fe to erythroid precursors will result in Fe deficiency, which results in anemia. Evolutionary advantage of hepcidin is that bacteria need Fe to grow and flourish. What are lab findings in ACD? ↑ferritin, ↓TIBC ↓serum iron (bone marrow takes Fe from serum as macrophage isn’t giving it), ↓% saturation ↑FEP (free erythrocyte protoporphyrin) (due to low available Fe but normal protoporphyrin, free protoporphyrin will ↑) What are stages of ACD? Normocytic anemia is followed by microcytic anemia (same as Fe deficiency anemia) What is treatment of ACD? Treat underlying cause of chronic disease (to reduce hepcidin) Exogenous erythropoietin (especially helpful in cancer pt) What are lab difference between Fe deficiency anemia and ACD? Fe deficiency anemia Anemia of chronic disease Hemochromatosis Oral contraceptive pill (OCP) ↓ ↓ ↑ Transferrin/TIBC ↑ ↓ ↓ Serum Fe ↑ Ferretin ↓ ↑ ↑ Transferrin/TIBC change is always opposite of ferretin. Look at ferretin first. TIBC (total iron binding capacity) is a measure of Transferrin Sideroblastic anemia (sidero = related to iron) What is pathophysiology of sideroblastic anemia? Low synthesis of protoporphyrin is main cause. If protoporphyrin is deficient, Fe is trapped in mitochondria. As mitochondria surround nucleus, Fe trapping presents as ringed sideroblasts. Fig: ringed sideroblasts seen in bone marrow biopsy (purssian blue stain – marks Fe) What are steps of heme synthesis? First and last three reactions take place inside mitochondria What are etiologies of sideorblastic anemia? Congenital Acquired Mutation of ALAS (most common cause of Alcoholism (EtOH is mitochondrial poison that congenital sideroblastic anemia) - XR damages protoporphyrin production) Lead poisioning (denatures ALAD and ferrocheletase) Vit B6 deficiency (ALAS requires Vit B6 as cofactor) – Isoniazid treatment can cause Vit B6 deficiency 10. What are clinical features of lead poisoning leading to sideroblastic anemia? Pt at old house with chipped paint at high risk Mnemonic LEAD: L - Lead lines on gingivae and metaphyses of long bones (aka Burton lines) E - Erythrocyte basophilic stippling and encephalopathy (lead inhibits rRNA degradation causing RBCs to retain aggregates of rRNA seen as basophilic stippling) A - sideroblastic Anemia, Abdominal colic D - Dimercaprol and EDTA for treatment; Wrist and foot Drop Succimer used for chelation in kids - sucks to be kid that eats lead Fig: From right to left - basophilic stippling; Burton lines on gum; metaphysis What are lab findings in sideroblastic anemia? What's its treatment? Lab findings based on Fe overload as they can’t attach to portoprophyrin As Fe increase in erythroblast, cells die due to free radical produced by Fenten reaction. Fe leaks out and is taken by macrophage. ↑ferritin, ↓TIBC ↑serum Fe, ↑% saturation Very similar lab findings as hemochromatosis Treatment: Pyrodoxine (B6 - cofactor for ALAS) Microcytic anemia 3 Tuesday, October 27, 2015 9:26 PM Thalassemia 1. What are normal globin molecule in hemoglobin? Hemoglobi abDevelopment n subuni subuni al stage t t HbF a Υ Fetal, persists for 6 moths after birth HbA α β Adult HbA2 α δ Adult (“Other adult”) 2. How does thalassemia lead to microcytic anemia? Thalassemia is decreased synthesis of globin chains. It results in reduced hemoglobin which leads to microcytic anemia. In sickle cell, there's defect in globin chain. What are patients with thalassemia protected against? Malaria by plasmodium falciparum. What causes alpha thalassemia? What chromosome is alpha gene located in? Alpha thalassemia is caused due to gene deletion of alpha chain of hemoglobin. Normally, 4 alpha alleles are present on chromosome 16 (2 allele per chromosome) 'a in alpha looks like d (deletion); if you rotate b in beta, you get m (mutation). What are subtypes of alpha thalassemia? 1 alpha allele deleted 2 alpha allele deleted Asymptomatic patient Severe anemia 4 alpha allele deleted - Hb Bart Hydrops fetalis Lethal in utero (hydrops fetalis) Cys deletion (deletion of both allele on same chromome) is worse than trans deletion (deletion of two allele on different chromosome) because cys is associated with increased risk of severe thalassemia in offspring No problem in fetus (one good alpha allele takes care) Cis deletion classically seen in Asians (higher rate of spontaneous abortion in Asia is partly due to this) Trans deletion classically seen in Africans When HbA and HbA2 are formed, B chain tetramers (aka HbH) are formed (4 B combine due to bad A) Gamma chain tetramers(aka Hb Barts) are formed HbH can be seen on electrophoresis Hb Barts seen on electrophoresis Mild anemia with slightly increased RBC count (remember in microcytic anemia, erythroblast divides more) 3 alpha allele deleted - HbH B chain tetramer What causes beta thalassemia? What chromosome is beta gene located in? Beta thalassemia is caused due to mutation of beta chain of hemoglobin. Mutations result in absent (aka B0) or diminished (aka B+) production of B-globin chain. Normally, 2 beta alleles are present on chromosome 11 (1 allele per chromosome) What are subtypes of beta thalassemia? B/B+ B0/B0 Aka Beta thalassemia minor Aka Beta thalassemia major Pt usually asymptomatic with increased RBC count Pt has severe anemia few months after birth as α4 tetramer will form (instead of α2β2) HbF at birth is temporarily protective (no B in fetal hemoglobin) Microcytic, hypochromic RBC and target cells on blood smear Hemoglobin electrophoresis findings: Increased HbA2 to 5% (normal 2.5%)- main finding Increased HbF to 2% (normal 1%) Slightly decreased HbA Microcytic, hypochromic target cells and nucleated RBC Hemoglobin electrophoresis findings: No HbA (no B chain) Increased HbA2 and HbF What are target cells? Normally, hemoglobin is present mainly on edge of RBC giving it biconcave shape with central pallor. In target cells, there are some hemoglobin in center giving central darkness (like bull's eye target practice). MOA- due to reduced hemoglobin in edge of RBC, the membrane in center gets floppy and some hemoglobin comes to stay there. What are presentation of beta thalassemia major? Bad erythropoiesis with severe anemia Extravascular hemolysis as spleen will phagocytose these RBC Massive erythroid hyperplasia o Expansion of hematopoiesis into skull and facial bone marrow - crew cut X-ray of skull and chipmunk face o Extramedullary hematopoiesis - hematopoiesis in liver and spleen (hepatosplenomegaly) o Risk of aplastic crisis with parvovirus B19 (parvovirus affects erythrocyte precursors and shuts down RBC production. In normal person, shutting down of RBC production for a week or so won't matter. For pt with beta thalassemia major, they can't afford even a single day of RBC production loss. They depend on every drop of RBC) Fig: chipmunk face (left) and crewcut appearance (right) seen in massive erythroid hyperplasia What is treatment of beta thalassemia major? What's it's complication? Treatment is chronic blood transfusion; splenectomy for swollen spleen and iron chelation to prevent secondary hemochromatosis Macrocytic anemia 1. What is macrocytic anemia? What are it's causes? Macrocytic anemia is anemia with MCV (mean corpuscle volume) >100. RBC precursor doesn't divide much and the RBC end up being big. Causes: o Megaloblastic anemia (anemia with big cells) - disruption in production of DNA precursors results in quick cytoplasmic development relative to nuclear development: Folate deficiency Vit B12 deficiency Orotic aciduria Folate and Vit B12 needed for DNA precursor synthesis o Alcoholism o Liver disease, drugs (ex- 5-FU) Megaloblastic anemia Vit B12 and Folate deficiency 2. Describe relationship between folate and Vit B12. Folate comes to body as methylated tetrahydrofolate (M-THF). THF is the active form. M-THF donates it's methyl group to Vit B12. Vit B12 then gives methyl group to homocysteine. Homocysteine now becomes methionine. What is presentation of macrocytic anemia due to folate or vit B12 deficiency? Megaloblastic anemia (impaired division of RBC precursors) Hypersegmented neutrophil with >5 lobes (normal is 3-5 lobes) (impaired division of granulocytic precursors) Megaloblastic changes in rapidly dividing cells (ex - intestinal epithelial cells) Fig: hypersegmented neutrophils with large RBC (aka macroovalocyte) on the left- classic finding in megaloblastic anemia What is difference between megaloblastic anemia and macrocytic anemia that's not megaloblastic? In macrocytic anemia that's not megaloblastic, hypersegmented neutrophils and megaloblastic changes (ex - large intestinal epithelial cells) won't be seen. Large RBC will be seen. Compare dietary information of folate and vit B12. Folate Vit B12 Food Dark green vegetable and food Animal derived proteins Absorption Jejunum Ileum Deficiency Develops in months as body stores are minimum Takes years to develop due to large hepatic storage Causes of deficiency Poor diet (alcoholics, old) Increased demand (pregnancy, cancer, hemolytic anemia) Folate antagonists (methotrexate - inhibits DHFR) Pernicious anemia (autoimmune destruction of parietal cells of stomach) most common Using proton pump inhibitor Pancreatic insufficiency (Vit B12 won't be free from R-binder) Damage to terminal ileum (Chron's, Diphylloborthium latum) Vegans (dietary deficiency rare otherwise) Compare clinical and lab findings of folate and Vit B12 deficiency. Folate deficiency Vit B12 deficiency Macrocytic RBC and hypersegmented neutrophils Macrocytic RBC and hypersegmented neutrophils Glossitis (inflammation of tongue - due to less turnover of tongue cells) Glossitis Low serum folate Low serum vit B12 Increased serum homocysteine (increases risk for thrombosis) Increased serum homocysteine (increases risk for thrombosis) Normal methylmalonic acid; no neuro symptoms Increased methylmalonic acid in myelin cells which impairs spinal cord myelinization resulting in subacute combined degeneration of spinal cord What are two important reactions that Vit B12 participate in? DNA precursor synthesis (with folate) Conversion of methylmalonic acid to succinyl Co. A Why do we see increased serum homocysteine in folate deficiency? Normally, dietary folate (M-THF) gives it's methyl group to Vit B12 which in turn gives it to homocysteine. Homocysteine now becomes methionine. The reaction won't happen in folate deficiency and we'll see increased serum homocysteine. Why will methylmalonic acid be increased in Vit B12 deficiency? Because Vit B12 is necessary to convert methylmalonic acid to succinyl Coenzyme A. Describe absorbtion of Vit B12 in gut (HY) R-binder protein from saliva binds to Vit B12. The complex will travel till small bowel. There's Vit B12 is set free by pancreatic proteases. The free Vit B12 binds to intrinsic factor secreted by parietal cells of stomach. This complex will go to ileum and get absorbed there. What are 3 P's of parietal cell? Proton pump - they pump proton to stomach to make it acidic Pink in histology (chief cells appear blue) Pernicious anemia if they get damaged - makes IF Orotic aciduria Defn Presentation Treatment Inability to convert orotic acid to uridine monophosphate (UMP) that leads to accumulation of orotic acid (defect in de-novo purine synthesis pathway) AR inheritence Megaloblastic anemia in children refractive to folate and vit B12 Failure to thrive, developmental delay Orotic acid in urine but no hyperammonemia UMP to pass the mutated enzyme Nonmegaloblastic macrocytic anemia Defn Macrocytic anemia where DNA synthesis is unimpaired RBC macrocytosis without hypersegmented neutrophils Causes Alcoholism Liver disease Hypothyroidism Reticulocytosis Normocytic anemia Wednesday, October 28, 2015 10:10 AM 1. What is normocytic anemia? What are two types based on etiology? Normocytic anemia is decreased RBC mass with normal-sized RBC (MCV - 80-100 µm3) Types: Peripheral destruction of RBC (will have reticulocyte >3%) Extravascular hemolysis (RBC destroyed by liver, spleen and lymph) Intravascular hemolysis (RBC destroyed within blood vessel) Underproduction of RBC (no increased reticulocytes) 2. What are reticulocytes? They are young RBC released from bone marrow to replace dead RBC Seen as large cells with bluish cytoplasm (due to RNA) on blood smear Normally, 1-2% of RBC die every day and are replaced by reticulocytes. 3. How can reticulocyte be falsely elevated in anemia? How is reticulocyte count corrected? Reticulocytes are measured as percent of total RBC. In anemia, total RBC goes down. It will elevating the percent of reticulocytes. It’s corrected by multiplying reticulocyte percent x hematocrit/45. Total RBC Total Reticulocyte % of reticulocyte Normal pt 100 (given) 2 (given) 2% Anemic pt 50 (given) 2(given) 4% HCT Corrected reticulocyte 23 (given) 4 x 23/50 = 2% In this example, if we only look at % of reticulocyte, it looks as if bone marrow is normal. But from corrected reticulocyte, we know that anemic pt’s bone marrow is not producing adequate reticulocytes. 4. How can reticulocyte count differentiate cause of anemia? If corrected reticulocyte >3% If corrected reticulocyte <3% Good marrow response (suggest peripheral destruction as cause of anemia) Poor marrow response (suggest underproduction of RBC as cause of anemia) Extravascular vs intravascular hemolysis 1. What is extravascular hemolysis? What happens to broken down RBC? Hemolysis done by reticuloendothelial system (macrophage in liver, spleen and lymph nodes) Globin is broken to AA; Iron is recycled Protoporphyrin is converted to unconjugated bilirubin which is carried by albumin to liver (its fat soluble). It’s conjugated in liver and excreted to bile. 2. What are lab and clinical finding of extravascular hemolysis? Anemia with splenomegaly Jaundice due to unconjugated bilirubin (too much bilirubin to be conjugated by liver) High risk for bilirubin gallstones Marrow hyperplasia with corrected reticulocyte >3% 3. What happens in intravascular hemolysis? What's the presentation? RBC is destroyed in blood vessels. Unlike macrophage breaking down hemoglobin to bilirubin, hemoglobin simply leaks out to blood. Hemoglobin is carried by haptoglobin. Haptoglobin is not present a lot. So, pt will quickly have hemoglobinemia and hemoglobinuria (hemoglobin water soluble) Hemosiderinuria after few days (HY) - hemoglobin in urine is picked up by renal tubular cells. Iron is recycled back and stored as hemosiderin. Renal tubular cells slough off (just like skin cells) and hemosiderin will be seen in urine. Presentation: Immediate Decreased serum haptoglobin Hemoglobinemia Hemoglobinuria After few days Hemosiderinuria Normocytic anemia with mainly extravascular hemolysis Wednesday, October 28, 2015 10:45 AM Hereditary spherocytosis 1. What is hereditary spherocytosis? What are the mutations? In the disease, tethering proteins that attach RBC cytoskeleton to RBC membrane are mutated. RBC membrane blebs and are lost over time. RBC becomes more spherical. Most common mutations are in proteins - ankyrin, spectrin, or band 3. 2. What are clinical and lab findings? See spherocytes - RBC becomes round instead of disc shaped (loss of central pallor) High RDW (some cells have lost tons of membrane and some only a little bit) high mean corpuscular hemoglobin concentration (MCHC) - high concentration of hemoglobin as cells are getting small Extravascular hemolysis findings o Anemia - spherocytes can't move through splenic sinusoids well and are eaten by splenic macrophages (this is main problem) - having spherocytes isn't bad o Splenomegaly (overworked spleen) o Jaundice with unconjugated bilirubin, high risk for bilirubin gallstones Fig: spherocytes with high RDW (note variability in RBC sizes and loss of central pallor) 3. What is one feared complication? Increased risk of aplastic crisis with parvovirus B19 infection of erythroid precursors 4. How is diagnosis of hereditary spherocytosis made? Osmotic fragility test - cells bursts in hypotonic solution very easily because cell doesn't have much membrane to expand out 5. What's it's treatment? Splenectomy (having spherocytes isn't problem, spleen eating them is problem) Anemia resolves but spherocytes persist and Howell-Jolly bodies are seen 6. What's Howell-Jolly bodies? Some RBC's are impefectly made with little nucleus or nuclear material left. It's job of spleen to take them out or kill the defective RBC. Howell-Jolly bodies are RBC with nuclear remnant. It indicates splenic dysfunction Fig: Howel Jolly bodies Sickle cell disease 1. What causes sickle cell anemia? It’s caused due to mutation in B chain of hemoglobin that changes glutamic acid (hydrophilic) to valine (hydrophobic). Think GingiVa - from Glutamic acid to Valine Disease is due to homozygous recessive mutation. Haterozygotes are protected against plasmodium falciparum malaria Phenotype Hemoglobin composition Sickle cell disease (homozygous mutation) 90% HbS, 8% HbF, 2%HbA2, no HbA Trait (one mutated and one normal B chain) 55% HbA, 43% HbS, 2% HbA2 HbS – sickle cell hemoglobin (in α2β2 protein, both copies of β are mutated) 2. What is pathogenesis of sickle cell anemia? HbS polymerizes when deoxygenated (reversible). The polymers accumulate into needle shaped structures and make RBC sickle cell. Sickling and de-sickling damages membrane leading to both intravascular and extravascular hemolysis (spleen eats damaged RBC); sickled RBC cause vaso-occlusion; massive erythroid hyperplasia to replace RBC. Sickling increases with hypoxemia, dehydration and acidosis. HbF protects against sickling. Kids protected for first few months of life. 3. What's treatment of sickle cell disease? Hydroxyurea - it increases level of HbF. It protects against sickling 4. What are presentations of sickle cell disease? Extravascular hemolysis – RBCs being sickle shaped and non-sickle cell repeatedly damages membranes. Reticuloendothelial system removes these damaged RBC. o Anemia o Jaundice with unconjugated hyperbilirubinemia o Increased risk for bilirubin gallstones Intravascular hemolysis – due to membrane damage o Decreased haptoglobin o Hemoglobinemia, hemoglobinuria o Hemosiderinuria after few days o Target cells - hemoglobin leaks out due to membrane damage and extra membrane produces target cells Massive erythroid hyperplasia (to compensate hemolysis and anemia): o Hematopoiesis in skull and facial bones (crewcut on X-ray and chipmunk face) o Extramedullary hematopoiesis (in liver, giving hepatomegaly - pt don't have spleen so don't get splenomegaly) o Risk of aplastic crisis with parvo B19 infection Fig: chipmunk face (left) and crewcut appearance (right) seen in massive erythroid hyperplasia Extensive sickling leads to vaso-occlusion 5. What are some physical findings in sickle cell disease due to vaso-occlusion? All findings based on infraction Dactylitis – due to vasoocclusive infaracts in bones – common in infants Autosplenectomy – shrunken, fibrotic and calcified spleen o Increased risk of encapsulated organism infection (staph aureus, strep pneumo, haemophilus influenza) o Salmonella paratyphi osteomyelitis (encapsulated) - most common cause of osteomyelitis is staph aureus; in sickle cell, it's salmonella. o Howel-Jolly bodies on blood smear - nucleated RBC Acute chest syndrome (vaso-occlusion of pulmonary microcirculation) o Often precipitated by pneumonia o Presents with chest pain, SOB, lung infiltrates Pain crisis Renal papillary necrosis – presents as gross hematuria and proteinuria Fig: vaso-occlusive complications of sickle cell disease - from left to right - autosplenectomy small calcified spleen; renal papillary necrosis; dactilytis; Howel-Jolly bodies 6. What’s the most common cause of death in sickle cell patients? Kids Hemophilus influenza infection Adults Acute chest syndrome 7. What is sickle cell trait? Haterozygote carriers of sickle cell mutation have sickle cell trait. They have one mutated and one normal beta chain. HbS (both beta chain mutated) makes <50% of total hemoglobin because HbA is slightly more efficiently made than HbS 8. What are presentations of sickle cell trait? Generally asymptomatic as RBC with <50% HbS don’t sickle Renal medulla problems: o Due to extreme hypoxia and hypertonicity in medulla, sickling occurs. o Presents as microscopic hematuria and decreased ability to concentrate urine due to microinfraction of medulla. 9. What are lab findings in sickle cell disease and trait? Sickle cell disease Sickle cell trait Sickle cell and target cells seen in blood smear Don’t see sickle cell or target cells Metabisulfite screen +ve (cells with any Metabisulfite screen +ve amount of HbS are sickled by the screen) Confirm amount and presence of HbS with Hb electrophoresis Sickle cell disease 90% HbS, 8% HbF, 2% HbA2, no HbA Trait 55% HbA, 43% HbS, 2% HbA2 Hemoglobin C 1. What is hemoglobin C? Hemoglobin C is formed due to mutation in Beta chain of hemoglobin (autosomal recessive). Glutamic acid is changed to lysine (lyCne for hemoglobin C) - think Gingiva - Glutamic acid to lyCine as gingiva is C shaped) Less common than sickle cell disease 2. What is presentation of hemoglobin C? Mild anemia due to predominant extravascular hemolysis HbC crystals on blood smear (HY) Fig: HbC crystals characteristic of hemoglobin C (the rods) Pyruvate kinase deficiency Pathophys Blood smear RBC depend on glycolysis to synthesize ATP Bad pyruvate kinase means ATP deficiency that affects lots of processes in RBC Echinocytosis (echino means hedgehog or sea urchin) - also see this in hyperlipidemia, uremia, hemolytic anemia, hypomagnesemia, hypophosphatemia etc Contrast Echinocytosis which looks similar to acanthocytosis Fig: acanthocytosis - see in hyperlipidemia or liver damage due to RBC membrane damage Normocytic anemia with mainly intravascular hemolysis Wednesday, October 28, 2015 7:04 PM Paroxymal nocturnal hemoglobinuria (PNH) 1. How do cells in blood protect themselves from complement system? DAF (decay accelerating factor) and MIRL (membrane inhibitor of reactive lysis) are present in RBC, WBC and platelets. They block complement fixation in RBC. DAF decays C3 convertase. Protein called GPI (glycosylphophatidylinositol) anchors MIRL and DAF to cells. 2. What causes paroxysmal nocturnal hemoglobinuria? It's acquired (not congenital mutation) defect in myeloid stem cell so that GPI is absent in myeloid stem cells. Complement fixation lyses RBC, WBC and platelets 3. What is presentation of PNH? Symptoms are seen paroxysmally at night because breathing becomes swallow and mild acidosis activates complement at night. Dark urine early morning Hemoglobinura, hemoglobinemia Hemosiderinura seen few days after hemolysis (after tubular cells slough off) Thrombosis - due to release of clotting factors from lysed platelets 4. What is main cause of death in PNH? Thrombosis of hepatic, portal or cerebral veins - due to release of clotting factors from lysed platelets 5. What are complications of PNH? Fe deficiency anemia (due to chronic loss of Hb in urine) Acute myeloid leukemia (10% of patients) 6. How is diagnosis of PNH made? Screening - Sucrose test Confirmatory test - acidified serum test or flow cytometry to test lack DAF (aka CD55) on RBC Glucose-6-Phosphatase dehydrogenase (G6PD) deficiency - aka favism 1. What is G6PD deficiency? What's it's pathophysiology X linked recessive disorder (see in men) that results in low half-life of G6PD. G6PD is first enzyme in pentose phosphate pathway and is required to make NADPH. NADPH is important to reduce oxidative stress. G6PD deficiency presents as increased oxidative stress including hemolytic anemia. 2. What are two major variants of G6PD deficiency? African variant Mediterranean variant Mildly reduced half-life of G6PD Markedly reduced half-life of G6PD Mild intravascular hemolysis with oxidative stress High intravascular hemolysis with oxidative stress 3. What protective role does being carrier of G6PD deficiency have? Protection against falciparum malaria 4. What's histology finding of G6PD deficiency anemia? Heinz bodies - precipitation of Hb due to oxidative stress Bite cells - Caused due to removal of Heinz bodies from RBC by macrophage 5. What are some causes of oxidative stress? Sulfa drugs Antimalarial drugs Fava beans 6. What's presentation of G6PD deficiency anemia? Hemoglobinuria and back pain (hemoglobin is nephrotoxic) hours after exposure to oxidative stress 7. What is diagnosis of G6PD deficiency? Screening - Heinz preparation - need to see heinz body Confirm - enzymatic studies (don’t do it during acute phase because RBC lacking G6PD are already dead). Immune hemolytic anemia (IHA) 1. What causes immune hemolytic anemia? IgG or IgM mediated destruction of RBC. 2. Differentiate between IgG vs IgM mediated IHA. IgG mediated IHA IgM mediated IHA Hemolysis is usually extravascular - tagged RBC are eaten Hemolysis is usually extravascular- tagged RBC are eaten Warm agglutination - IgG binds to RBC in warm temp (central parts of body). Splenic macrophage phagocytose tagged RBC leading to formation of spherocytes (when RBC are eaten only halfway, remaining RBC makes sphere) Cold agglutination - IgM binds to RBC in cold temp (extremities). RBC can inactivate complement, but C3b acts as opsonin for splenic macrophages - see spherocytes Associated with: Lupus - pt have anti-blood Ab CLL (chronic lymphocytic lukemia) - cause hemolytic anemia drugs (classically penicillin and cephalosporins) - drug induces autoantibody production or Ab can bind to drugRBC complex Associated with: Mycoplasma pneumoniae (cold agglutination test) infectious mononucleosis (+ve haterophile agglutination - Ab made against sheep blood) CLL Treatment Stop offending drug Steroids IVIG (distract spleen) Splenectomy - spleen is the one that eats RBC 3. How do you diagnose IHA? Direct coombs test Indirect coombs test Confirms presence of Ab or complement coated RBC Confirm presence of anti-RBC Ab in patient's blood When anti-IgG or anti-complement Ab are added to pt RBC, agglutination occurs only if RBC are already coated with IgG or complement Anti IgG and test RBC(normal RBC) are mixed in patient serum (agglutination occurs only if serum Ab are present) Most important test for IHA Microangiopathic hemolytic anemia 1. What is microangiopathic hemolytic anemia (hemolysis in small blood vessel)? It's hemolysis that occurs due to vascular pathology (usually something in blood vessel breaks the RBC) 2. What are some etiologies? Presence of microthrombi o TTP- thrombotic thrombocytopenic purpura o HU- hemolytic uremic syndrome o DIC o HELLP - hemolysis elevated liver enzyme and low platelet Prosthetic heart valves - crush RBC Aortic stenosis - crush RBC 3. What is blood smear finding? Schistocytes (broken RBC) - aka helmet cells Fig - schistocytes (helmet cells) - has mostly two acute angle and loss of about 50% of RBC; contrast bite cells that have usually >2 acute angles and almost entire volume of RBC is present. Malaria 1. How does malaria cause anemia? Plasmodium infects and replicates in RBC. RBC ruptures as merozoites (a stage in their lifecycle) are released Spleen also consumes infected RBC causing some extravascular hemolysis Erythroblastosis fetalis Maternal IgG crossing placenta and attacking fetal RBC (ex - Rh -ve mother carrying two consecutive Rh +ve babies) Present See extramedullary hematopoiesis (ex - in liver) because RBC are damaged Defn Normocytic anemia due to underproduction Wednesday, October 28, 2015 8:24 PM 1. What is anemia due to underproduction? It's anemia caused due to low RBC production by bone marrow. Characterized by low corrected reticulocyte (<3%) 3. What are some etiologies of anemia due to underproduction? Renal failure - decreased erythropoietin production by peritubular interstitial cells Anything that causes microcytic and macrocytic anemia Damage to bone marrow precursor cells - ex parvovirus B19 4. Describe how parvovirus B19 infection leads to anemia. Parvovirus B19 infects progenitor RBC and temporarily halts erythropoiesis. It causes significant anemia in setting of preexisting marrow stress (ex - sickle cell) Treatment is supportive (infection is self-limited) Aplastic anemia 1. What is aplastic anemia? Aplastic anemia is damage to hematopoietic stem cell resulting in pancytopenia (anemia, leukopenia, thrombocytopenia) 2. What are etiologies of aplastic anemia? Etiologies: o Drugs or chemicals, radiation o Viral infection - parvo B19, HIV, EBV, HCV o Autoimmune damage o Fanconi anemia (inherited DNA repair defect that causes bone marrow failure) - high risk of leukemia later 3. What are biopsy finding in aplastic anemia? Empty fatty marrow Fig: Aplastic anemia (left) vs normal bone marrow on right. Note the depletion of marrow and replacement by fat globules on left. 4. What's treatment for aplastic anemia? Immunosuppression for cases with abnormal T cell activation Stop causative drugs Blood transfusion and marrow stimulating factors (erythropoietin, GM-CSF, G-CSF) May need bone marrow transplant Myelophthisic process 1. What are melophthisic process? Pathologic processes that replace bone marrow (ex - cancer) Hematopoiesis is impaired resulting in pancytopenia Lymphoid tissue anatomy Saturday, December 5, 2015 2:57 PM 1. Spleen PALS (periarteriolar lymphatic sheath) - has T cells (drink tea with pals) - in white pulp Germinal center - has B cells - in white pulp