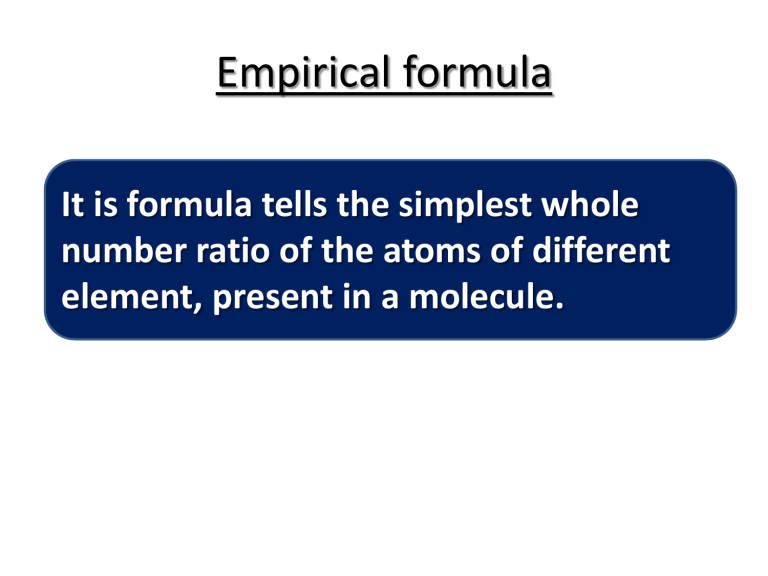
Empirical formula It is formula tells the simplest whole number ratio of the atoms of different element, present in a molecule. Empirical formula C6 H 12 O6 C8 H10 N4 O2 C6 H6 S2 Cl2 C12 H22 O11 C2 H2 C5 H 10 O5 H2O2 Calculation of empirical formula Q1.A sample of copper metal weighing 2.50 g is heated to form an oxide of copper. The final mass of the oxide is 3.13 g. Determine the empirical formula of the oxide. Elements Masses Divide by Ar Divide by smallest ratio Simplest whole numbers Empirical formula Calculation of empirical formula Q2. In the analysis of 2.500 g of an unknown compound, it is found that 0.758 g are calcium, 0.530 g are nitrogen and 1.212 g are from oxygen. Find the empirical formula and the identity of this compound Calculation of empirical formula Q3. A 0.338 g sample of antimony was completely reacted with 0.295 g of chlorine gas to form antimony chloride. Determine the empirical formula of the antimony chloride. Ar Sb 122 Calculation of empirical formula Q4 A sample of the black mineral hematite, an oxide of iron found in many iron ores, contains 34.97 g of iron and 15.03 g of oxygen. What is the empirical formula of hematite? Calculation of empirical formula Q5. Chemical analysis shows that citric acid contains 37.51% C, 4.20% H, and 58.29% O. What is the empirical formula? Calculation of empirical formula A sample of copper is heated in a crucible to form black copper oxide, the following data is collected Mass of crucible = 35.75g Mass of crucible + copper = 37.12g Mass of crucible + copper oxide = 37.47g a.Calculate mass of copper used b.Calculate mass of oxygen that have reacted with copper. c. Find the empirical formula of copper oxide Calculation of Molecular formula Q7 . What is the molecular formula of a compound with a percent composition of 49.47% C, 5.201% H, 28.84% N, and 16.48% O, and a molecular mass of 194.2 amu? n= molecular mass empirical formula mass Molecular formula = n (empirical formula) Q8 . Nicotine, an alkaloid in the nightshade family Calculation of Molecular formula of plants that is mainly responsible for the addictive nature of cigarettes, contains 74.02% C, 8.710% H, and 17.27% N. If molecular mass of Nicotine is 162.3g. what is the molecular formula of Nicotine? Calculation of Molecular formula Q9 . Methyl ethanoate is a pleasant smelling chemical, composed of C, H and O. It has following composition C = 48.65%, H = 8.11%, O= 43.24% . Its relative molecular mass is 74. Find the molecular formula of this compound. Calculation of Molecular formula Q10. A 1.00 g sample of one of these compounds contains 0.040 g of hydrogen, 0.242 g of carbon and 0.718 g of chlorine. (i) Calculate the empirical formula of this compound [2] (ii) The relative molecular mass of the compound is 99 .Deduce the molecular formula of the compound. [1] CH2Cl C2H4Cl2 Calculation of Percentage composition Ar and Mr or formula mass can used to calculate the percentage by mass of an element in a compound. % of an element by mass = Mass of element X 100 Mr of compound Calculation of Percentage composition 1. Calculate percentage composition of CH3COOH Calculation of Percentage composition 2. Calculate percentage by mass of Carbon in C3H6O 3. Calculate percentage by mass of each element in C4H8O2 Calculation of Percentage composition 4. Formula of Sodium sulphate decahydrate is Na2 SO4 . 10H2O a. Calculate percentage by each element. b. Calculate percentage of water . Calculation of Percentage composition 4. Formula of Copper sulphate pentahydrate is CuSO4 . 5H2O a. Calculate percentage by mass of copper. b. Calculate percentage of water . C. Calculate percentage by mass of CuSO4