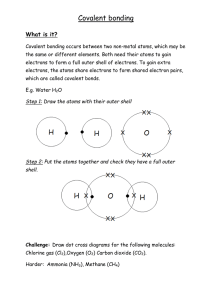
Introduction to ionic bonding SC5a Ionic bonds Welcome to BONDING This will be your new topic for the next few weeks. So get ready! What’s in the Bonding topic? - Ions Ionic Bonding Ionic Structures & Properties Simple covalent bonding Giant covalent structures Metallic Bonding Starter: Keywords Compound Element Mixture Bond Paired Task - complete these definition for each keyword - no devices! Compound - two or more elements . . . . . . . . Element - substance made up of only . . . . . . . Mixture - substance containing two or more . . . . . . . . Bond - forces of attraction that . . . . . . . . Task 1: Keywords Compound Element Mixture Bond Paired Task - complete these definition for each keyword - no devices! Compound - two or more elements joined together Element - substance made up of only one type of atom Mixture - substance containing two or more different substances that are not joined together Bond - forces of attraction that hold atoms together What are the different types of bonding? You need to know the three different types of bonding that exists between atoms. Different types of bonds are formed depending on the types of atoms involved: We will learn this one first ● Ionic bonding – occurs between metal and non-metal atoms. ● Covalent bonding – occurs between non-metals atoms only. ● Metallic bonding – occurs between metal atoms only. BIG QUESTION How can atoms become stable? Why do atoms form bonds? Atoms are more stable if they have an outer electron shell that is FULL, like a noble gas. What is an ion? Electrons can transfer between atoms with incomplete outer electron shells. Metals tend to give away outer shell electrons and non-metals tend to gain electrons. An ion is an atom or group of atoms that has an electrical charge, either positive and negative. Forming POSITIVE ions An atom that loses electrons has more protons than electrons and so has a positive overall charge. This is called a positive ion. Consider a sodium atom, which has one electron in its outer shell: A sodium ion has a full outer shell which noble gas also has this electron configuration? Forming POSITIVE ions Sodium atom: 11 protons = +11 11 electrons = -11 Total charge = 0 Na Sodium ion: 11 protons = +11 10 electrons = 10 Total charge = +1 loses 1 electron 2.8.1 (partially full outer shell) - + Na [2.8] (full outer shell) Forming POSITIVE ions Magnesium 12 protons +12 12 electrons 12 Total charge 0 atom: = = = Magnesium ion: 12 protons = +12 10 electrons = 10 Total charge = - +2 2+ Mg loses 2 electrons 2.8.2 (partially full outer shell) Mg [2.8]2+ (full outer shell) Task 1: Aluminium Aluminium is a metal. It has 13 electrons. In your exercise book draw two diagrams, showing: 1. the electronic structure of an aluminium atom 2. the electronic structure and charge of an aluminium ion Forming NEGATIVE ions An atom that gains electrons has more electrons than protons and so has a negative overall charge. This is called a negative ion. Consider a fluorine atom which has 7 electrons in its outer shell: Forming NEGATIVE ions Fluorine atom: 9 protons = +9 9 electrons = -9 Total charge = 0 Fluoride ion: 9 protons = +9 10 electrons = 10 Total charge = -1 - F 2.7 (partially full outer shell) gains 1 electron F [2.8] (full outer shell) Forming NEGATIVE ions Sulfur atom: 16 protons = +16 16 electrons = 16 Total charge = - 0 S gains 2 electrons 2.8.6 (partially full outer shell) Sulfide ion: 16 protons = +16 18 electrons = 18 Total charge = -2 S [2.8.8]2 (full outer shell) 2 - Task 2: Oxygen Oxygen is a non-metal. It has 8 electrons. In your exercise book draw two diagrams, showing: 1. the electronic structure of an oxygen atom 2. the electronic structure and charge of an oxide ion Kahoot! Let’s test your understanding! https://create.kahoot.it/share/y10introduction-to-ionic-bonding/0bf3b3e8-9cdd4d5c-a337-5e4c946745a3