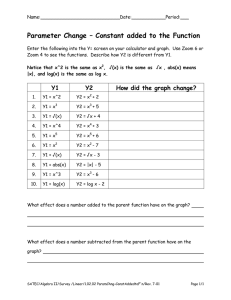
13. Lewis Dot Symbols Name:______________________ Pd:____ Directions: Fill in the simplified periodic table with the elements’ Lewis Dot Symbol. Look at hydrogen as an example of how to fill out the table. Groups 1 2 13 (3A) 14 (4A) 15 (5A) 16 (6A) 1 17 (7A) 18 (8A) Atomic Number → 2 1 ABS: 1 LP: 0 ABS = # of Available Bonding Sites, LP = # of Lone Pairs → ABS: __ LP:__ 3 4 5 6 7 8 9 10 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 11 12 13 14 15 16 17 18 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 19 20 31 32 33 34 35 36 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 37 38 49 50 51 52 53 54 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 55 56 81 82 83 84 85 86 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 87 88 113 114 115 116 117 118 ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ ABS: __ LP:__ 2 3 4 5 6 7 1. What patterns did you notice in each group? (Hint: talk about valence electrons, ABS, and LP) 2. Draw the Lewis dot symbol for oxygen. Label the available bonding sites and lone pairs. 3. Draw the Bohr model for oxygen. 4. Compare and contrast the Lewis dot symbol and Bohr model you drew for oxygen. a. What are the similarities? b. What are the differences? 5. Losing electrons to form ions a. Which groups will lose electrons to get a full octet? _______________________ b. For each group you listed, tell me how many electrons they will lose and what charge they will have once they lose those electrons. 6. Gaining electrons to form ions a. Which groups will gain electrons to get a full octet? _______________________ b. For each group you listed, tell me how many electrons they will gain and what charge they will have once they gain those electrons. Bonus* For a piece of candy. 7. A water molecule has the chemical formula H 2O. This means that two hydrogen atoms are bonded to an oxygen atom. a. Draw two Lewis dot diagrams for hydrogen and one for oxygen. b. Show how these hydrogen atoms bond to the oxygen atom.