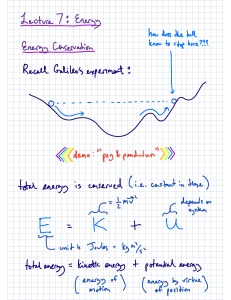
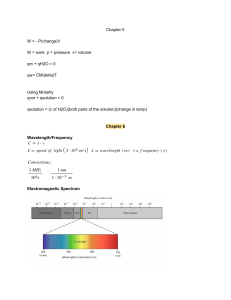
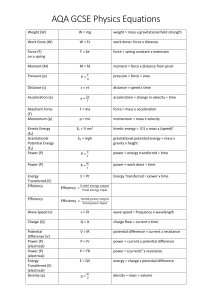
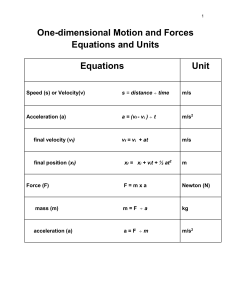
Thermodynamics: Equation Sheets: Chap 1: number of moles m n v M v M Temperature Conversions: R 1 .8 K F R 4 5 9 .6 7 C K 2 7 3 .1 5 F 1 .8 C 3 2 --------------------------------------------------------------------------------------------------------------Chap 2: Energy balance of closed system Energy Rate balance of close system dE U KE PE Q W Q W dt Work due to action of a force Work due to compression of a fluid r2 W V2 F dr F W V r1 pdV V1 Kinetic Energy KE 1 mV Gravitational Potential Energy PE m gz 2 2 Energy Balance: Power Cycle Power Cycle Efficiency W c y c le Q in Q o u t W c y c le Q in Energy balance: Ref and Heat Pump Refrigeration COP W c y c le Q in Q o u t Heat Pump COP Q in W c y c le Q out W c y c le -----------------------------------------------------------------------------------------------------------------Chap 3: Quality Polytropic Process v v f x (v g v f ) x m vapor u u m liq u id m v a p o r f x (u g u f ) pV N c o n s ta n t h h f x (hg h f ) Ideal Gas Model: pv RT pV m RT or U m cv d T m cv T c p cv R cp kR k 1 or pV nRT H m c p dT m c pT cv R k 1 Compressibility Model: pv Z pv RT p pR RT TR pc Chap 4: Continuity Equation: AV m v T v 'R Tc v R Tc / p c Mass balance for CV: V dm v dt mi i me e Energy Balance for CV d E cv Q cv W cv dt Vi m i ( hi 2 i Simplified Nozzle/Diffuser model: 2 gzi ) m e ( he Ve e 2 2 gze ) Simplified Turbine model: Vi Ve 0 hi he 2 2 2 2 0 W cv m ( hi he ) Simplified Compressor/Pump model Simplified Throttling model 0 hi he 0 W cv m ( hi he ) Simplified Heat Exchanger model 0 mi i 0 me m i hi i e m e he e ----------------------------------------------------------------------------------------------------Chap 5: 2nd Law Efficiency 2nd Law COP m ax 1 TC m ax TH TC m ax T H TC TH T H TC Clausius Inequality: Q T b c y c le ------------------------------------------------------------------------------------------------------Chap 6: Closed System Entropy Balance Closed System Entropy Rate Balance 2 S 2 S1 Q T b 1 dS c y c le Control Volume Entropy Balance dS dt Q T m i si m e se dt Q T Isentropic Efficiencies: Turbine tu r b in e W cv / m W cv /m s Nozzle h1 h 2 Compressor/Pump 2 n o z z le h1 h 2 s V2 / 2 co m p ress 2 (V 2 / 2 ) s W cv /m s W cv / m h 2 s h1 h 2 h1 Ideal Gas Model Relations: s ( T 2 , v 2 ) s ( T 1 , v 1 ) c v ln T2 R ln T1 v2 s ( T 2 , p 2 ) s ( T1 , p 1 ) c p ln v1 T2 R ln T1 p2 p1 or s ( T 2 , p 2 ) s ( T1 , p 1 ) s ( T 2 ) s ( T1 ) R ln o o p2 p1 Isentropic Ideal Gas relationships (when s1 = s2) p2 T1 p1 ( k 1 ) / k v1 T1 v2 T2 k 1 T2 p2 p1 p2 p1 pr2 v2 p r1 v1 v1 v2 k vr 2 v r1 -------------------------------------------------------------------------------------------------------------------