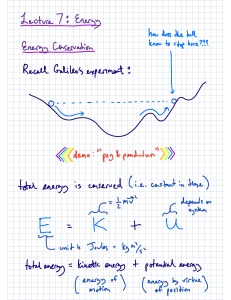
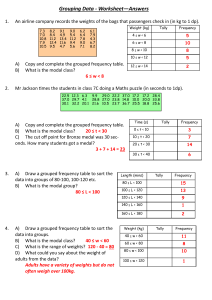
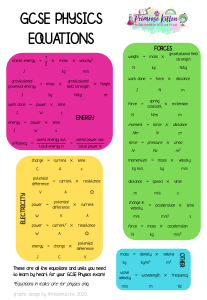
Chapter 5 W = - P(change)V W = work p = pressure v= volume qm + qH2O = 0 qw= CM(delta)T Using Molarity qrxn + qsolution = 0 qsolution = (c of H2O)(both parts of the solution)(change in temp) Chapter 6 Wavelength/Frequency Electromagnetic Spectrum Energy of Light: Quantitative Steps Hydrogen Atom: Quantitative Ionization of H+ = infinity . Emission ↓ Absorption ↑ Long wavelength = low frequency = high energy transition = short line Short wavelength = high frequency = low energy transition = long line Matter Waves Quantum Numbers n= Principal l= Angular Momentum Orbital Shapes m1 = orbital orientation ms= -½, +½ Sublevels and electrons Electron Configuration & Exceptions Valence electron Configuration ● Higher Volume of n ○ Ignore d block Ion electron Configuration a Atomic size Ion Size A Isometric Ions A