Entropy & Ideal Gas Processes: Thermodynamics Module
advertisement
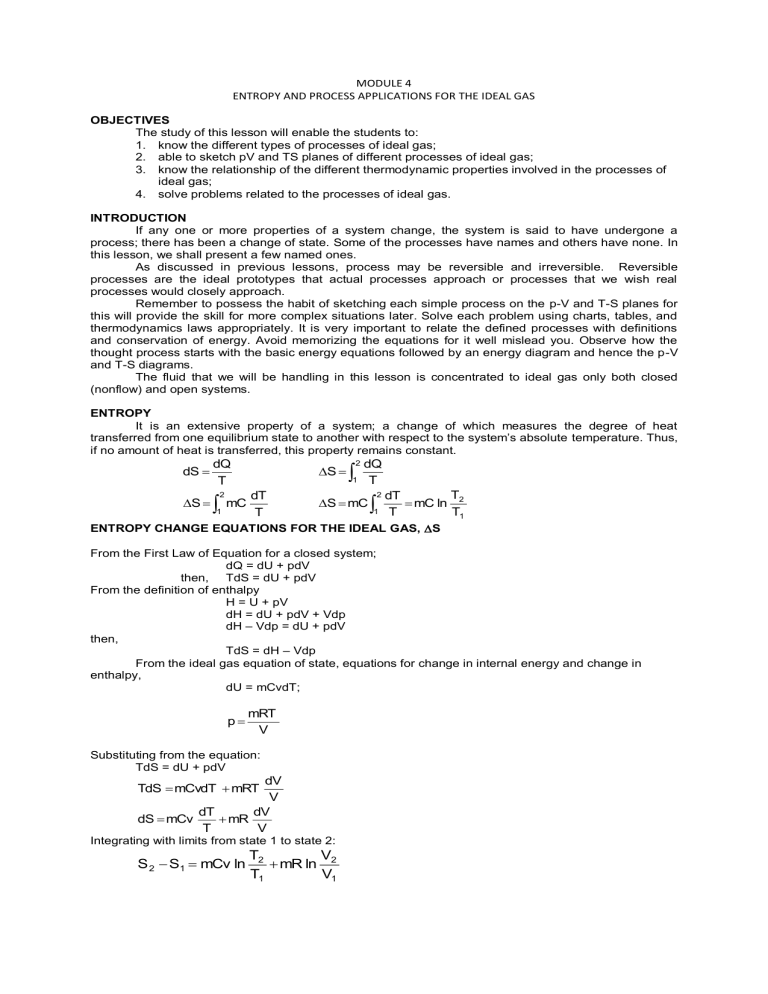
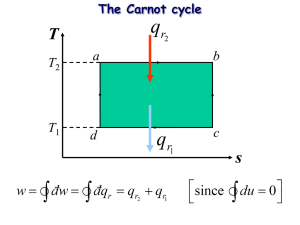
MODULE 4 ENTROPY AND PROCESS APPLICATIONS FOR THE IDEAL GAS OBJECTIVES The study of this lesson will enable the students to: 1. know the different types of processes of ideal gas; 2. able to sketch pV and TS planes of different processes of ideal gas; 3. know the relationship of the different thermodynamic properties involved in the processes of ideal gas; 4. solve problems related to the processes of ideal gas. INTRODUCTION If any one or more properties of a system change, the system is said to have undergone a process; there has been a change of state. Some of the processes have names and others have none. In this lesson, we shall present a few named ones. As discussed in previous lessons, process may be reversible and irreversible. Reversible processes are the ideal prototypes that actual processes approach or processes that we wish real processes would closely approach. Remember to possess the habit of sketching each simple process on the p-V and T-S planes for this will provide the skill for more complex situations later. Solve each problem using charts, tables, and thermodynamics laws appropriately. It is very important to relate the defined processes with definitions and conservation of energy. Avoid memorizing the equations for it well mislead you. Observe how the thought process starts with the basic energy equations followed by an energy diagram and hence the p-V and T-S diagrams. The fluid that we will be handling in this lesson is concentrated to ideal gas only both closed (nonflow) and open systems. ENTROPY It is an extensive property of a system; a change of which measures the degree of heat transferred from one equilibrium state to another with respect to the system’s absolute temperature. Thus, if no amount of heat is transferred, this property remains constant. dS dQ T S 2 1 mC S dT T 2 1 dQ T S mC 2 1 T dT mC ln 2 T T1 ENTROPY CHANGE EQUATIONS FOR THE IDEAL GAS, S From the First Law of Equation for a closed system; dQ = dU + pdV then, TdS = dU + pdV From the definition of enthalpy H = U + pV dH = dU + pdV + Vdp dH – Vdp = dU + pdV then, TdS = dH – Vdp From the ideal gas equation of state, equations for change in internal energy and change in enthalpy, dU = mCvdT; p mRT V Substituting from the equation: TdS = dU + pdV dV V dT dV dS mCv mR T V TdS mCvdT mRT Integrating with limits from state 1 to state 2: S 2 S1 mCv ln T2 V mR ln 2 T1 V1 S 2 S1 mCv ln dH mCpdT T2 V mR ln 2 T1 V1 V also mRT P Substituting values in TdS = dH – Vdp dp p TdS mCpdT mRT dS mCp dT dp mR T p Integrating with limits from state 1 to state 2 T2 p2 mR ln ; kJ / K T1 p1 T2 p2 s2 s1 Cp ln R ln ; kJ / kg.K T1 p1 S2 S1 mCp ln THE SECOND LAW AS APPLIED TO ENTROPY The second law of thermodynamics is an expression of fact that all forms of energy are not necessary equivalent in their ability to perform useful work. Entropy Explains the Second Law An isolated system’s entropy never decreases. If all processes that occur within the system are reversible, its entropy remains constant; otherwise, its entropy increases. Entropy can be produced but never destroyed As entropy is produced the ability to do useful work decreases. Note: The entropy of a system is the sum of the entropies of its components IDEAL GAS PROCESSES Six Thermodynamic Processes Constant-Volume Process (Isometric or Isochoric Process) Constant-Pressure Process (Isobaric or Isopiestic Process) Constant-Temperature Process (Isothermal Process) Constant-Entropy Process (Isentropic Process) Constant Enthalpy Process (Throttling or Isenthalpic Process) Polytropic Process CONSTANT VOLUME PROCESS (ISOMETRIC OR ISOCHORIC) An isometric process is a reversible constant volume process. A constant volume process may be reversible or irreversible P P2 T 2 2 ΔU V=C P1 1 1 V V 1 = V2 Q Q S S1 S2 P-V and T-S ISOMETRIC PROCESS a) Relationship between Pressure and Temperature P1 P2 T1 T2 b) Reversible Non Flow Work 2 W nf pdV 0 1 c) The change in Internal Energy U mCv T2 T1 d) The Heat Transferred Q mCv T2 T1 e) The change in Enthalpy H mCp T2 T1 f)The change in Entropy S mCv ln T2 T1 g) Reversible steady flow constant Volume Process From the General Energy Equation of Steady flow process Q U PE KE E f Wsf h) Irreversible Non-flow constant Volume Process From the General Energy Equation of Steady flow process Q U PE KE Wn Take Note: For reversible non-flow process, For Irreversible non-flow process, Wn = 0 Wn 0 Example: 1. A perfect gas has a value of R = 58.8 ft-lb/lboR and k = 1.26. If 20 BTU are added to 5 lb of this gas at constant volume when the initial temperature is 90 oF, Find (a) T2; (b) ∆H; (c) ∆S; (d) ∆U; and (e) Work for a non flow process. 2. A reversible, non flow, constant volume process decreases the internal energy by 316. kJ for 2.268 kg of gas for which R = 430 J/kg.K and k = 1.35. For the process, determine (a) the work, (b) Q, and (c) S. The initial temperature is 204.4 oC. CONSTANT PRESSURE PROCESS (ISOBARIC OR ISOPIESTIC) An isobaric process is an internally reversible process of a substance during the pressure remains constant. W T P 1 2 T2 2 V=C V=P 1 T1 V V1 ΔU Q Q S S1 S2 V2 P-V and T-S of ISOBARIC a) Relationship between Pressure and Temperature V1 V2 T1 T2 b) Reversible Non Flow Work 2 W nf pdV p( V2 V1 ) 1 c) The change in Internal Energy U mCv T2 T1 d) The Heat Transferred Q mCpT2 T1 e) The change in Enthalpy H mCp T2 T1 f)The change in Entropy T S mCp ln 2 T1 g) Reversible steady flow constant Pressure Process From the General Energy Equation of Steady flow process Q U PE KE E f Wsf h) Irreversible Non-flow constant Pressure Process From the General Energy Equation of Steady flow process Q U PE KE Wn Example: 1. Three pounds of a perfect gas with R=38 ft-lb/lboF and k= 1.667 have 300 BTU of heat added during a reversible nonflow constant pressure change of state. The initial temperature is 100oF. Determine the (a) final temperature, (b) ∆H, (c) W, (d) ∆U and (e) ∆S 2. While the pressure remains constant at 689.5 kPa the volume of a system of air changes from 0.567 m3 to 0.283 m3. What are (a) ∆U, (b) ∆H, (c) Q, (d) ∆S and (e) If the process is non flow and internally reversible, what is the work? CONSTANT TEMPERATURE PROCESS (ISOTHERMAL) An isothermal process is an internally reversible constant temperature process of a substance P T 1 1 2 pV=C 2 Q pdV V V1 S S 1 V2 S P-V and T-S of ISOTHERMAL PROCESS2 a) Relationship between Pressure and Volume P1V1 P2 V2 b) Reversible Non Flow Work 2 Wnf pdV 1 2 V CdV p1V1 ln 2 V V1 1 c) The change in Internal Energy U 0 d) The Heat Transferred Q U Wn P1V1 ln V2 P mRT ln 1 V1 P2 e) The change in Enthalpy H 0 f)The change in Entropy S P Q mR ln 1 T P2 g) Reversible steady flow constant Temperature Process From the General Energy Equation of Steady flow process Q U PE KE E f Wsf h) Irreversible Non-flow constant Volume Process From the General Energy Equation of Steady flow process Q U PE KE Wn Example: 1. Four pounds of air gain 0.491 BTU/oR of entropy during the a non flow isothermal process. If P1 = 120 psia and V2 = 42.5 ft3, find (a) V1, (b) T1 (c) W, (d) Q, and (e) ∆U. 2. If 10 kg/min of air are compressed isothermally from P1= 96 kPa and V1 = 7.65 m3/min to P2 = 620 Kpa, find the work, the change of entropy and the heat for (a) non flow process and (b) a steady flow process with υ1 = 15 m/s and υ2 = 60 m/s. CONSTANT ENTHALPY PROCESS (THROTTLING OR ISENTHALPIC PROCCESS) A throttling or isenthalpic process is an irreversible adiabatic process, where no work is done. Whatever a fluid flows in a steady flow manner from the higher-pressure region to a lower pressure region with no work performed, then the process is said to have undergone throttling. It should be noted that the throttling process, or any so-called free expansion, is not organized for the production of work or for conserving the kind of energy that might be converted into work (as in kinetic energy generated in a nozzle.) The steady-flow equation then reduces to KE1 + h1 = KE2 + h2 If the Kinetic energy is assumed to be equal, then the equation becomes; dh = 0 h1 = h 2 or Cp(T2-T1) This equation indicates that the throttling process of an ideal gas though at constant temperature, is not an isothermal process. Boyle’s Law applies for a throttling process of an ideal gas using either equation; p1V1 = p2V2 ΔS = m R ln also, V2 ; V1 ΔS = m R ln p1 p2 and the change in entropy is always greater than zero. Throttling process analysis could be well explain if attention will be in process of vapor system. CONSTANT ENTROPY PROCESS (ISENTROPIC) An isentropic process is a reversible adiabatic process, being adiabatic indicates that there is no heat transfer for this process. T P 1 1 pVK=C pV= C 2 pdV 2 S V V1 S1=S2 V2 P-V and T-S of ISENTROPIC a) Relationship between Pressure and Volume PROCESS k P1V1 k P2 V2 C b) Relation between Temperature and Volume P1V1 k P2 V2 and k T2 V1 T1 V2 P1V1 P2 V2 T1 T2 k 1 c) Relation between Temperature and Pressure T2 P2 T1 P1 k 1 k b) Reversible Non Flow Work p1V1 C, p CV k k 2 2 W nf pdV CV k dV C 1 1 2 1 V k dV Integratin g and Simplifyin g P V P V (T T1 ) Wn 2 2 1 1 mR 2 1 k 1 k c) The change in Internal Energy U mCv T2 T1 d) The Heat Transferred Q0 e) The change in Enthalpy H mCp T2 T1 f)The change in Entropy S 0 g) Reversible steady flow constant Isentropic Process From the General Energy Equation of Steady flow process Q PE KE H Wsf a) Wsf PE KE H if PE 0, KE 0 Wsf H 2 b) Vdp Wsf KE 1 1 let C p k V or V Cp 2 1 2 Cp Vdp 1 k 1 k 1 Integratin g and simplifyin g, 2 1 Vdp 2 k p 2 V2 p1V1 k pdV 1 1 k h) Irreversible Non-flow constant Volume Process From the General Energy Equation of Non-flow process Q U PE KE Wn Example: 1. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.63 ft3 to a final volume of 3.6 ft3. If cp = 0.124 and cv = 0.093 BTU/lboR, what are (a) t2, (b) P2, (c) ∆H, (d) W. 2. A certain ideal gas whose R=278.6 kJ/kg and cp = 1.015 kJ/kg.K expands isentropically from 1517 kPa, 288oC to 965 kPa. For 45 kg of this gas, determine (a) W n, (b)V2, (c) ∆U and (d) ∆H. POLYTROPIC PROCESS A polytropic process is a quasi-static and internally reversible process during which pV n C and p1V1 p 2V2 p iVi n n n where n is any cons tan t P T 1 1 pVn=C pVn=C 2 pdV 2 V V1 S S1 V2 P-V and T-S of a Polytropic Process Q S2 a) Relationship between Pressure and Volume P1V1 n P2 V2 C n b) Relation between Temperature and Volume P1V1 n P2 V2 and n T2 V1 T1 V2 P1V1 P2 V2 T1 T2 n1 c) Relation between Temperature and Pressure T2 P2 T1 P1 n 1 n b) Reversible Non Flow Work p1V1 C, p CV n n 2 2 W nf pdV CV n dV C 1 1 2 1 V n dV 2 V n1 cons tan t 1n cons tan t V2 V11n n 1 1 n 1 P2 V2n V21n P1V1n V11n 1 n Simplifyin g P V P V (T T ) Wn 2 2 1 1 mR 2 1 1 n 1 n c) The change in Internal Energy U mCv T2 T1 d) The Heat Transferred Q U Wn mC v T2 T1 mR T2 T1 1 n C nC v R T2 T1 m v 1 n Cp nCv T2 T1 but Cv R Cp kCv m 1 n (k n) T2 T1 m Cv (1 n) m Cn T2 T1 where Cn often called the polytropic specific heat e) The change in Enthalpy H mCp T2 T1 f)The change in Entropy S mCn ln T2 T1 g) Reversible steady flow constant Polytropic Process From the General Energy Equation of Steady flow process Q PE KE H Wsf a) Wsf Q PE KE H if PE 0, KE 0 Wsf Q H 2 b) Vdp Wsf KE 1 1 let C p n V or V Cp 2 1 Vdp 2 1 Cp 1 n 1 n Integratin g and simplifyin g, 2 2 np 2 V2 p1V1 Vdp n pdV 1 1 1 n h) Irreversible Non-flow constant Volume Process From the General Energy Equation of Non-flow process Q U PE KE Wn Example: 1. A polytropic process of air from 150 psia, 300 oF, and 1 ft3 occurs to p2 = 20 psia in accordance with pV1.3 = C. Determine (a) t2, (b) V2,(c) ∆U, (d) ∆H, (e) ∆S, (f) non flow work, (g) steady flow work for∆K = 0. 2. The work required to compress a gas reversibly according to pV 1.30=C is 67,790 J, if there are no flow. Determine ∆U and Q if the gas is (a) air, (b) methane. For methane, k = 1.321, R=518.45 kJ/kg.K, cv=1.6187, cp=2.1377. CURVES FOR CHOOSING VALUES OF n. Polytropic processes are all inclusives in that many of the prior equations can be obtained by choosing proper values of n. Let n = 0; then pv0 = C of p = C, an isobaric process Let n = ∞; then, from pVn = C, we have p1/nV = p1/∞V = V = C, an isometric process Let n=k; then pVk=C, an isentropic process Let n=1; then pV=C, and isothermal process Note: The isentropic curve on the pV plane is steeper than the isothermal curve and on the TS plane the constant volume curve is steeper than the constant pressure curve when both are drawn between the same temperature limits. Effect of Varying n. Expansions are imagined to take place from some common point 1. Notice that all positive values of n give curves in the second and fourth quadrants on the pV plane (a) that positive values of n may produce curves in all four quadrants on the TS plane, (b) Notice too that curves with values of n between 1 and k will fall in the second and fourth quadrants on the TS plane and within the narrow region on the pV plane. v s IDEAL GAS FORMULAS For constant mass systems undergoing internally reversible processes Process Isometric Isobaric Isothermal V=C P=C T=C p, V, T relations T2 p 2 T1 p1 T2 V2 T1 V1 p1V1 = p2V2 Isentropic S=C Polytropic pVn = C p1V1k = p2V2k p1V1n = p2V2n T2 V1 T1 V2 k 1 k 1 2 pdV 0 p(V2 – V1) V(p2 – p1) 0 1 2 Vdp 1 V2 V1 V p1 V1 ln 2 V1 p1 V1 ln T2 p 2 k T1 p1 p 2 V2 p1 V1 1 k k p 2 V2 p1 V1 1 n n 1 T2 V1 T1 V2 n 1 T2 p 2 n T1 p1 p 2 V2 p1 V1 1 n n p 2 V2 p1 V1 1 n 2 m CvdT n pdV 1 U2 – U1 2 2 m CvdT m CvdT mCv (T2 – T1) mCv (T2 – T1) 1 2 2 m CvdT m CpdT mCv (T2 – T1) mCp (T2 – T1) n ∞ 0 Specific Heat, C Cv Cp H2 – H1 m CpdT m CpdT mCp (T2 – T1) mCp (T2 – T1) Q S2 – S1 1 1 2 1 CvdT 1 T T mCv ln 2 T1 m 2 CpdT 1 T T mCp ln 2 T1 1 mCv (T2 – T1) 2 2 1 mCv (T2 – T1) 2 m CndT m TdS 1 1 0 mCn (T2 – T1) 1 k -∞ to +∞ ∞ 0 kn Cn Cv 1 n k C 0 m CpdT m CpdT mCp (T2 – T1) mCp (T2 – T1) p1 V1 ln V2 V1 2 1 m m CvdT 0 1 2 2 1 Q T mR ln 2 1 CndT 1 T T mCn ln 2 T1 m V2 V1 0 2 2