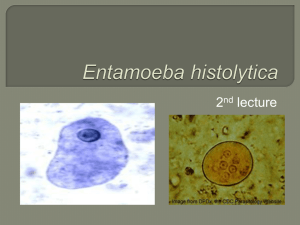
OVERVIEW OF DIAGNOSTIC PARASITOLOGY AND SPECIMEN COLLECTION Diagnosis of parasitic infections through demonstration of parasite or parasite components (adults, egg, larvae, cysts, trophozoite) Specimens received in the Lab: Stool – most common Aspirates (Duodenal, Liver) Blood, Buffy Coat and Lymphatic Fluid Urine • Eye Scrapings, Skin Scrapings • Biopsy Specimens • Other body fluids (i.e. CSF, Vaginal Fluid, Amniotic Fluid) 1. Stool • Most common method • Demonstration of eggs, larvae, adults, trophozoites, cysts, or oocysts in the stool • Best collected in: • clean, wide-mouthed containers made of waxed cardboard or • plastic with a tight-fitting lid to ensure retention of moisture and to prevent accidental spillage • Properly Labeled; submitted together with a lab request • Important Factors to be considered • Intake of drugs/ medicinal substances 1. Antacids 4. Bismuth 2. Anti-diarrheals 5. Laxatives 3. Barium •Stool Examination should be deferred •Amount of Stool Submitted A routine stool examination usually requires: • a thumb-sized specimen of formed stool • about 5 to 6 tablespoons of watery stool. •Contamination with toilet water, urine, or soil must be prevented since these can destroy protozoan trophozoites. In addition, soil and water may contain free-living organisms that would complicate diagnosis of infections •Stool Processing and Handling • Watery/Diarrheic Stool: examine within 30 minutes from time of passage • Formed stool: up to 24 hours • Temporary storage of fecal samples in a refrigerator (3-5°C) is acceptable • Trophozoites are killed by refrigeration, although helminth eggs and protozoan cysts are usually not damaged. • NEVER FREEZE STOOL SAMPLES. NEVER KEEP THEM IN INCUBATORS • Fixatives • Ratio: 3 parts preservative to 1-part stool specimen • Fixation Time: 30 minutes 1.Formalin 4.SAF 2.Merthiolate Iodine Formalin 5.Modified PVA 3.PVA (combined with Schaudinn’s) 6. Alternative Single Vial System Ova and Parasite Examination 1. Macroscopic Examination 2. Microscopic Examination Techniques • Direct Fecal Smear • Kato-Thick Smear • Concentration Techniques • Kato-Katz Techniq • Stoll Dilution Technique • Preparation of Permanent Stained Smears – confirmation of intestinal protozoan o Use of Iron Hematoxylin or Trichrome •Macroscopic Examination • Physical Characteristics • Color • Consistency or Form of Stool • Gross Abnormalities Microscopic Examination 3 Distinct Procedures: • Direct Wet Preparations • DFS • Concentrated Wet Preparations • Sedimentation – ex. FECT, AECT • Flotation – ex. Zinc Sulfate Flotation (1.18-1.20); Sheather’s Sugar Flotation • Permanently Stained Smear • Trichrome, Iron Hematoxylin, Specialized Stains • Presence of Cysts, Eggs, Adult forms, larval forms, trophozoite stages • Fecal Elements that might be mistaken as Parasites > Leukocytes > Vegetable Hair > Muscle Fibers > Fat Droplets > Vegetable Cells > Fungal, Yeast Cells > Vegetable Spiral • Charcot Leyden Crystals: eosinophil breakdown product > Significant Stool Specimens • Other Procedures • Cultures - Harada Mori Technique, Coproculture & Use of Culture Media • Egg Counting Procedure - Kato-Katz (Cellophane Covered Thick Smear) & Stool Egg Count Other Specimens from the intestinal tract and urogenital system • Collection of Perianal Swab - Scotch Tape Swab or Cellulose Tape Swab • Sigmoidoscopy Material • Duodenal Contents- Duodenal Drainage & Duodenal Capsule Technique (Entero-Test) 2. Blood • Thick and Thin Smears - for malaria • Knott’s Concentration technique – for microfilaria • Buffy Coat Smear: for hemoflagellates 3. Sputum Parasites that may be recovered on sputum: • Migrating larvae: (ASH) Ascaris lumbricoides, Strongyloides stercoralis, and hookworms • Paragonimus ova • Echinococcus granulosus hooklets from pulmonary hydatid cysts • Protozoa such as: • Entamoeba histolytica trophozoites from pulmonary amebic abscess • Cryptosporidium parvum oocysts, although very rare • Non-pathogenic Entamoeba gingivalis and Trichomonas tenax • First morning specimen best • Patient cannot expectorate > use inductants (10% sodium chloride or hydrogen peroxide) 4. Urine • First morning specimen best since there could have been concentration of parasites overnight • Best for Trichomonas vaginalis • May also detect Wuchereria bancrofti and Schistosoma haematobium 5. Tissue Aspirates • Sample aspirated from the ff organs: • Liver • Duodenum • Skin • Bronchial • Lymph node • Liver aspirate: most common in the Philippines • To rule out hepatic amoebic abscess • For diagnosis of Echinococcus granulosus in endemic areas • Duodenal aspirate: uses String test • Cutaneous or Skin aspirates : For Cutaneous Leishmaniasis (Oriental sore) • Cerebrospinal Fluid: •Trypomastigotes of Trypanosoma cruzi, Trypanosoma brucei rhodesiense, and Trypanosoma brucei gambiense • trophozoites of Naegleria • Parastrongyliasis • Specimen examined within 20 minutes • Tissue biopsy : For Trichinella spiralis • Rectal biopsy : Presence of deposited eggs of Schistosoma japonicum 6. Animal Inoculation/Xenodiagnosis • Animal Inoculation • Xenodiagnosis • Uses arthropod vectors or other hosts as an indicator of infection • Used in diagnosis of Chagas’ disease and Trichinosis PROTOZOANS Amebae MEDICALLY IMPORTANT PARASITES • Protozoans • Helminthes • Nematodes • Trematodes • Cestodes PROTOZOANS (KINGDOM PROTISTA) a. Phylum Sarcomastigophora • Subphylum Sarcodina – Ameba • Subphylum Mastigophora – Flagellates b. Phylum Ciliophora – Ciliates c. Phylum Apicomplexa • Class Sporozoa • Suborder Haemosporina d. Suborder Eimeria PROTOZOANS (SARCODINA) Taxonomy of Protozoans Kingdom Protista • Phylum Sarcomastigophora o Subphylum Sarcodina o Subphylum Mastigophora • Phylum Ciliophora • Phylum Apicomplexa • Phylum Microspora (now fungi) PROTOZOANS • Unicellular Organisms • Vary in shape, size, locomotion • Reproduce Sexually or Asexually • Do not possess a cell wall • Consists of Nucleus and Cytoplasm • Nucleus: Genetic Material Contains nucleolus or karyosome or endosome • Cytoplasm 2 Regions: Endoplasm & Ectoplasm • Trophozoite a. • • • • • STAGES OF DEVELOPMENT • Cyst Subphylum SARCODINA Possess Pseudopodia for locomotion Inhabit the large intestine except E. gingivalis All undergo encystation except E. gingivalis All undergo Binary Fission as mode of reproduction All are commensals except E. histolytica Sarcodina • • Pathogenic: Entamoeba histolytica Commensals: o E. dispar o E. moshkovskii o E. hartmanni o E. coli o Endolimax nana o Iodamoeba butschlii 1. Entamoeba histolytica • Entamoeba histolytica – only pathogenic member • MOT: Ingestion of Infective Cyst • Habitat: Large Intestine • Final Host: Man Entamoeba coli vs E. histolytica Categories Movement Pseudopodia Nucleus of Trophozoites and location of karyosome E. histolytica Trophozoite Unidirectional One at a time on an explosive manner Finger- like Blunt; E. coli Sluggish; Not progressive; Several at a time but slow Rounded Mononucleated Central karyosome CYST Red Blood Cells (hematophagous) Mono Eccentric karyosome Cytoplasm No. of Nuclei (Cyst) Clean looking 1-4 or 4 Dirty looking 1-8 or more than 4 Chromidial bars/ bodies Sausage shaped; rod-cigar shaped; coffin with rounded ends Fine and evenly distributed Broom stick needles; splintered glasses; witch broom Irregular, clamp, coarse Inclusions Peripheral chromatin PATHOGENESIS Bacteria, yeast, debris E. histolytica Mechanisms for virulence: 1. Asymptomatic – majority of • production of enzymes cases or other cytotoxic • Excrete cysts 2. Intestinal Disease substances • contact-dependent cell • Incubation Period 1-4 weeks killing • Release of enzymes to lyse • cytophagocytosis mucosal lining DISEASE MANIFESTATIONS • Ameboid Movement a. Asymptomatic Carrier • Formation of FLASK SHAPED ULCERS Clinical Forms of Intestinal Amebiasis State – majority of cases • Dysentery – majority of cases b. Intestinal Disease • Amebic colitis (abdominal pain + diarrhea +/- blood & (amebic colitis, mucus in the stools ) ameboma) • Fulminating Colitis (seen in children) c. Extra-intestinal • Amebic Appendicitis Disease – Hepatic • Ameboma 3. Extra-intestinal Disease • Ectopic form of amebiasis • Usually occurs in the Liver >>> Amebic Liver Abscess • Cardinal Signs: Fever, Right Upper Quadrant Pain • Other signs include: tender liver and hepatomegaly Drainage of a liver abscess Chronic amebiasis: drainage of a lung abscess Chronic amebiasis: brain abscess PATHOLOGY • • Ability to lyse tissues Attributed to its Virulence Factors: o Lectin (Gal/GalNAc Lectin) o Amebapores o Cysteine Proteinases LABORATORY DIAGNOSIS • Ova and Parasite Examination o DFS o Concentration Techniques o Permanent Stained Smear (Iron Hematoxylin or Trichrome Stain) Charcot Leyden crystals • • • • • • Culture o Boeck’s, Rice Egg Saline, Diamond, Balamuth’s Egg Yolk Infusion Serology (ELISA) Molecular Methods (PCR) Rectal Biopsy Examination of Liver Aspirates Ultrasound, CT scan, MRI EPIDEMIOLOGY • • • • Worldwide Distribution More Prevalent in Tropics High Risk Groups Issues on the occurrence of a “non-pathogenic” E. histolytica o Recent identification of a “E. histolytica look-a Treatment • • • Metronidazole: Tx of acute amebic colitis Diloxanide Furoate: Asymptomatic Cyst Carriers Iodoquinol PREVENTION • • • • Proper Disposal of Waste Proper Sanitation/personal hygiene Access to safe water and food Development of an effective Vaccine E. histolytica a. Pathology: Invasiveness and abscess formation are due to amoebic proteolytic enzymes b. Immunology: Antibodies are detectable in chronic infections but they are of questionable protective value Diagnostic features E. histolytica and some non-pathogenic amoebae Amebiasis Differential diagnosis: Amebiasis is different from giardiasis and bacterial dysentery. (Mucus and blood in stool, No granulocytosis, No high fever) 2. E. dispar and E. moshkovskii are morphologically similar to E. histolytica but they are non-pathogenic Other Commensal Amebae: Generally, do not cause disease and their life Cycle is similar to Entamoeba histolytica 3. Entamoeba coli 4. Entamoeba hartmanni is a small Race of E. histolytica (Commensal) 5. Entamoeba polecki is an Ameba of pigs and monkeys (Entamoeba species) Iodamoeba butschlii is an Ameba of swine (pigs) Prominent Feature: • Uninucleated with a large eccentric karyosome; with achromatic granules “Basket of Flowers” • Large glycogen vacuole 6. 7. Endolimax nana is the smallest intestinal amebae (as small as a RBC) (Commensal) 8. Entamoeba gingivalis • Ameba of oral cavity (Gum Line) • No cystic stage • MOT: Person to Person • Infective and Diagnostic Stage: Trophozoite • Scavengers and eat debris; can ingest WBCs, debris, RBC(rare) • Non-pathogenic; but seen in patients with pyorrhea alveolaris FREE LIVING PATHOGENIC AMEBAE • Found inhabiting lakes, pools, tap water, air conditioning units and heating units • In Humans: Found in the CNS o Acanthamoeba o Balamuthia o Naegleria 1. Naegleria fowleri • Free-living Ameboflagellate • MOT: Entry into the body: Olfactory Epithelium, Respiratory Tract, Skin and Sinuses Important stages • • Cyst Trophozoite – infective stage Ameba – feeding form Flagellate – swimming form Entry into the body Disease Manifestation and Pathology olfactory epithelium, respiratory tract, skin and sinuses Primary Amebic Meningoencephalitis • VERY FATAL • Risk Factor: Swimming in contaminated pools, lakes and rivers Pathogenic determinant PRESENCE OF Amebostomes Diagnosis • Wet Mount Examination of CSF • Smears stained with Wright’s or Giemsa • Biopsy • Culture • Molecular Methods Treatment and Prevention Amphotericin B with Clotrimazole 2. Acanthamoeba spp. • Free-living Ameba • Characteristic Feature: Presence of acanthapodia • Morphologic Forms: Cyst Disease Manifestation and Pathogenesis Disease Manifestation Acanthamoeba • Causes Granulomatous Amebic Encephalitis Chronic; slow in progression Poor Prognosis Amebic Keratitis • Keratitis, uveitis, Corneal Ulcerations • Implicated among contact lens users Trophozoite Diagnosis • Usually diagnosed after death (GAE) • Biopsy • Corneal Scrapings • Culture • Molecular Methods Treatment • Very Fatal once cerebral manifestations appear • Fluorocystine, ketoconazole, amphotericin B 3. Balamuthia • Granulomatous Amebic Encephalitis (GAE) • Found in soil and water • 1st discovered in 1986 in the brain of a mandrill that died in the San Diego Wild Animal Park