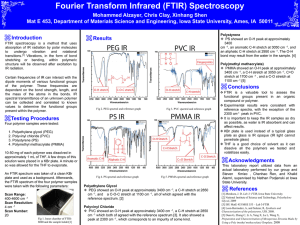
Introduction Fourier-transform infrared spectroscopy is known as FTIR. FTIR technique can use to characterize the both natural and synthetic polymers. For the FTIR analysis it uses the infra-red radiation in the electromagnetic wave spectrum. The size of the infra-red radiation is 10-5 and it has higher energy. The machine that uses to analyse the polymer characteristics is called spectrophotometer. In the FTIR graph it has unique number range for the chemical bonds. FTIR spectrophotometer Figure 1:FTIR spectrophotometer FTIR spectrophotometer is the instrument that use to analyze the functional groups of polymers. This machine has two modes, one is ART and other one is transmittance mode. This instrument uses the IR beam to analyze the polymer functional groups. IR radiation has high energy and it causes to stretch or bend of the functional group’s bonds. The IR absorption of the polymer generates the infra-red spectra of the polymer. The reflection glasses of the FTIR spectrophotometer is made with KBr because of those materials don’t absorbers the infra-red radiations. This is very useful for the reverse engineering process. Procedure Transparent sample 1. First spectrophotometer was turned on. 2. After that Unknown Sample A, B and C were taken to test. 3. First the Unknown Sample A sample was taken and placed on the sample holder and it was placed in the spectrophotometer sample holder. 4. Then the machine was calibrated using the software by taking background data. 5. After that the sample was tested. 6. Finally, the generated data was recorded and stored in the storage device. 7. Above same procedure was repeated to the Unknown B and C samples. Readings Figure 2: FTIR graph of Unknown Sample A Figure 3: FTIR graph of Unknown Sample B (PVCP Figure 4: FTIR graph of Unknown Sample C (Acitic Acid) Discussion FTIR is known as Fourier-transform infrared spectroscopy. It a important test in reverse engineering and functional group of polymer can be identify using the FTIR. It can detect the infra-red radiation that absorbed by the organic test specimen. All organic molecules absorb radiation in the infra-red range of wavelengths. The generated graph can be identified using below illustration. Figure 5: Graph of FTIR According to Figure 5 graph is generating as Absorbance of the IR energy by sample vs Wavenumber. The Y axis is Absorbance and the X axis is Wavenumber. The energy of the IR wave (X axis) is increasing from the left to right. The blue color circled pointes are the peaks of the graph. Peaks are the points that helps to identify the molecules which are testing. When applying IR energy to molecules it starts to vibrate the molecules. The graph generated according to the vibration. In FTIR graph of Unknown Sample A (Figure 2) it has weak and narrow peak in range of 2969 and that means it has Carbon and Hydrogen (C-H). This sample also has a benzene group and it shows clarify C-C stretches in the wavenumber range of 1504 to 1760.. Also, in this spectrum it has strong peak value in range of 1600 to 2000 and that means it has Carbon, Oxygen (C-O) double bond. According to those predictions this sample should be polyethyleneteripthalen (PET). In FTIR graph of Unknown Sample B (Figure 3) it shows medium peaks in wavenumber range of 2849 and 2915. Because of this peak it can predict Unknown polymer B has alkane C-H stretching. The Medium narrow peaks in wavenumber of relatively 730 and it means this polymer has C-Cl stretching. Unknown polymer sample B should be PVC. In FTIR graph of Unknown Sample C (Figure 4) it has broad peak in wavenumber range of 3200 to 3600 which belongs to the O-H stretching. This broad peak is very broad and overlapped with the C-H stretching. And it is belongs to the C-O double bond. With that peaks this polymer (Unknown sample C) should be acetic acid. References Rajapaksha SM, 2020, ‘Unit 02: Fourier-transform infrared spectroscopy (FTIR)’ lecture notes distributed in the topic natural rubber latex products, University of Sri Jayewardeneprure, on 04th September 2020. Department of Chemistry and Biochemistry, Typical IR Absorption Frequencies For Common Functional Groups. NORTHERN ILLINOIS UNIVERSITY. https://www.niu.edu/clas/chembio/research/analytical-lab/ftir/ir-frequencies-table.shtml; accessed 2021.12.30