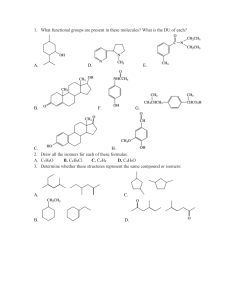
SI Exam 1 Review Handout Chapter 1 Formal Charge: Give the formal charge of each charged atom in the molecule below: Hybridization: For each of the indicated atoms below, give the hybridization state of the atom, its molecular geometry, and its approximate bond angles. 1 Constitutional Isomers: For each pair of molecules below, state whether the molecules are constitutional isomers, the same molecule, or neither. A. Constitutional Isomers B. Same Molecule C. Unrelated Molecules (Not Isomers) A. Constitutional Isomers B. Same Molecule C. Unrelated Molecules (Not Isomers) A. Constitutional Isomers B. Same Molecule C. Unrelated Molecules (Not Isomers) 2 Resonance: Draw all possible resonance structures for the following molecules and select the major contributor. 3 Chapter 2 Functional Groups: Circle and identify each of the functional groups present in the following molecules. Codeine Heroin Melting/Boiling Point: Which of the following molecules would have the highest boiling point? A) B) C) D) HOCH2CH2OH CH3COCH3 CH3CH2CH2OH CH3CH2CH2F Rank the following molecules in order of boiling point. Do the same for melting point. 4 Solubility: Which of the following molecules is most likely to dissolve in hexane? A) B) C) D) E) Ethanol CCl2H2 CH3OCH3 (CH3)2CHCH2CH3 NH3 Which of the following molecules is most likely to dissolve in water? A) B) C) D) E) BH3 CCl4 CH3COOH CO2 CH3CH2CH3 5 Infrared Spectroscopy: Which IR spectrum correlates to each of the following compounds? Compound:______ Compound:______ Compound:______ Compound:______ 6 Which of the following molecules will give a C-H stretching signal below 3000 cm-1? (Hint: There may be multiple answers) Which of the following molecules will give a sharp peak between 2100 and 2300 cm-1 and a sharp peak at 3300 cm-1? (Hint: There may be multiple answers) Which of the following molecules will give a sharp peak between 2100 and 2300 cm-1 but no sharp peak at 3300 cm-1? (Hint: There may be multiple answers) 7 Which of the following molecules most likely gave the IR spectrum given below? IR Spectrum: Chapter 3 Acid-Base Strength: Choose the stronger acid in each pair. 8 Choose the stronger base in each pair. Gibb’s Free Energy and Leveling Effect: For each reaction below, determine if the ∆G° for the reaction in the forward direction will be negative or positive. How about the Keq? Would the reaction be spontaneous? 9 10