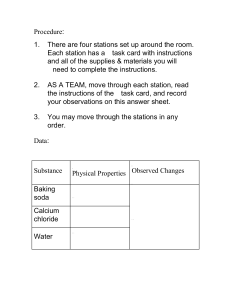
Name ______________________________________ Date___________ Period ___________ Temperature La Supplie 1 250mL beake 100mL graduated cylinde 3 ice cube 2 teaspoons of baking sod 150mL distilled vinega Stopwatc _ . . . a r r b r s h s I I I Procedure: Part 1. Fill one beaker with 150 mL of water 2. Place a thermometer in the beaker and time 1 minute. Record the temperature in Data Table 1. This is the beginning temperature 3. Place 3 ice cubes in the beaker. **DO NOT STIR THE WATER WITH THE THERMOMETER.** Read the temperature every minute for 5 minutes and record your readings in the data table. You should have 6 readings now **DO NOT STIR THE WATER WITH THE THERMOMETER.** Part I 4. Pour 150 mL distilled vinegar into a 250 mL beaker. **DO NOT STIR THE WATER WITH THE THERMOMETER.** After 1 minute, read the temperature of the vinegar. This is the beginning temperature. 5. VERY SLOWLY add 1 teaspoon of baking soda into the beaker. After one minute, observe the temperature in the beaker. Record the temperature in Data Table 2. 6. VERY SLOWLY add another teaspoon of baking soda to the beaker. Observe and record the temperature every minute for a total of 4 more minutes. You should now have 6 readings in Data Table 2. 7. After collecting your data in Celsius, complete Data Table 1 and 2 by converting your temperatures to Fahrenheit by following the rule F = (C x 1.8) + 32 8. Use the information in Data Table 1 and 2 to graph the change in temperature over time on the graph paper provided Part II 10. Answer the followup questions. Data Table Time Temperature in C Temperature in F Temperature in C Temperature in F Beginning temperature 1 minute (ice) 2 minutes (ice) 3 minutes (ice) 4 minutes (ice) 5 minutes (ice) Data Table Time Beginning temperature 1 minute (baking soda) 2 minutes (baking soda) 3 minutes (baking soda) 4 minutes (baking soda) 5 minutes (baking soda) 2 1 Data Table 1 Data Table 2 Follow Up Questions 1. How can we monitor the change of temperature of a liquid over time 2. Which rate of temperature change was faster, Part I or Part II 3. How does the graphing of the data re ect the rate of temperature change in Part I and Part II 4. What do you predict may have happened if you had added a third teaspoon of baking soda to the vinegar ? ? fl ? ? 5. Discuss one way scientist may use graphs to evaluate temperature change over time.