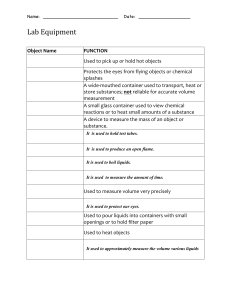
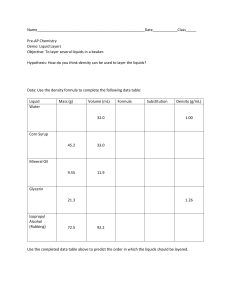
SCIENCE FAIR 2021 Density column 12/12/2021 Date: Name: Arppana Kantharupan Bavanuja Kugachanthiran Aim Hypothesis Aim - The aim of this experiment is to stack different liquids to visually demonstrate how can an object sink or float From a list of solutions, predict which arrangement will have the highest density and which arrangement will have the lowest density - the less dense liquid floats on top of the more dense liquid) What is Density Coloumn? A density column is a holder of fluids stacked in layers. The layers stay separate on the grounds that every substance has an alternate density from the others. All in all, weighty fluids have more mass or matter per unit of volume than lighter liquids. How is a density column used to determine density? Density = Mass divided by Volume. In light of this situation, if the weight (or mass) of something increments however the volume remains something similar, the density needs to go up. Moreover, if the mass reduces, yet the volume remains something similar, the density needs to go down. How does the density column work? The first layer you pour stays at the lower part of the glass since it has the most elevated thickness or measure of mass per unit volume. The last layer you pour has the most reduced thickness. A few fluids, similar to oil and water, don't blend since they repulse one another. Different fluids, similar to honey and maple syrup, don't promptly blend since they have distinctive consistency levels (thickness or capacity to stream). Be that as it may, after some time, a few fluids in the segment will combine as one. Risk Assesment Spilling the liquid solutions, this may cause skin or eye irritation - wear safety glasses and gloves to protect Students ought not ingest any of the investigation There are no particular security risks related with any of the synthetic compounds utilized in this test Emergency treatment – wash the impacted region with water. Standard consume emergency treatment ought to be kept Materials & Ingredients Honey (from the bottom of the cylinder) Maple syrup Dish soap Water (coloured) Oil (add 2 drops of colour) Nail polish remover (this is to the top of the cylinder) Variables Independent variable different kind of liquids Dependent variable - the layers of the liquids with different density Constant variable - the amount of each liquids that is formed in a cylinder Procedure Set up your liquids. Use food shading to color fluids, whenever wanted. It's useful to arrange liquids all together before you start. You will pour from the first spot on the list (generally dense) and work your direction to the lower part of the rundown (least dense). Carefully empty the initial liquid into the lower part of the holder. Attempt to try not to contact the side of the glass. This first liquid is thick, so it's difficult to pour. One procedure is to set a spoon in the holder. Pour onto the spoon so the fluid streams down it, as opposed to bringing down the side of the glass. Add the following liquid. Either pour it down the spoon, as in the past, or utilize a baster or pipette to gradually convey the fluid to the highest point of the main layer. One tip is to rest the spoon or baster tip on the glass simply over the highest point of the layer. This helps prevent accidental mixing. Ensure each layer is genuinely thick, so it will appear regardless of whether a little mixing happens. Keep adding liquids as such until you're finished. Presently, you can respect your work or use it as an embellishment. A well-constructed density column keeps up with its layers for a few hours or days. When pouring the liquid , pour it from the edge of the cylinder Results state the outcome of the experiment and the Discussion Discussion Our experiment was reliable and accurate by using the density formula of D=m/v and taking measurements of the volume Conclusion - explain the outcome; discuss whether it proves or disproves hypothesis My hypothesis was successful because honey was at the bottom of the cylinder when calculating the density using the formula D = m/v (density= mass/volume), we got the highest volume for the honey