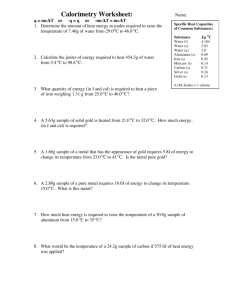
Name: _ _ _ _ _ _ _ _ _ _ _ _ Period: Score: _ _ __ Calculating Heat Worksheet Substance Water Grain Alcohol Ice Wood Steam CWorofonn Aluminum Iron Silver Men:un· G-Old Specific Heat (.J/2°C) 4.18 2.40 2.10 1.80 1.70 1) A 122.0 gram sample of water is raised 65.4°C. How much heat was added to the system? 0.96 o.90 0.46 0.24 0.14 0.128 2) 916 J of energy are removed from a sample of iron. If it cools from 60.0°( to 12.8°(1 what is the mass of the sample of iron? 3) A 181 gram sample of metal cools 82.0°c. If 13,360 J of energy are released, what type of metal is it? 4) A sample of silver releases 45.6 kJ of energy. If the sample has a mass of 421 240 grams, what was the drop in temperature? 5) What is the specific heat of a substance if 476 J of energy are released lowering a 283 gram sample 12.0°C? Which substance is it? 6) 472 J of energy are absorbed by wood raising the temperature 18.2°C. What is the mass of the sample? 7) Ice absorbs 8.2 kJ of energy. If the sample is 72.2 g and the original temperature is -121°(, what is the new temperature? 8) A 1.902 kg sample of grain alcohol absorbs a large amount of heat which raises the temperature from 20.5°( to 77.0°c. How much heat was absorbed? 9) A sample of steam absorbs 883 kJ of energy and increases its temperature from 112°C to 235°c. What is the mass of the sample of steam? 10) A 180.0 g sample of chloroform sits on a shelf. If the temperature in the room is raised from 17.0°c to 33.2°c, how much heat did the chloroform absorb? 11) A 422 g sample releases 162.6 kJ of energy and drops 92.2°c. What is the specific heat of the substance? And what is the substance? 12) A 17.0 g sample of iron absorbs 330.0 J of energy. If the original temperature was 18.0°c, what is the new temperature? 13) A 2.88 kg sample of ice gains 3024 J of energy. If the original temperature is -o.5°C, what is the new temperature? Heat Lost Heat Gained 14) A metal shard with a mass of 23.0 g is heated to a temperature of 162.9°C. It is dropped into 50. g of water. The water was originally 55°C and it heats to 64.7°C. What is the specific heat of the metal and what is the identity of the metal? 15) A silver bracelet is stored in a deep freeze. The 152 g silver bracelet is placed in a 232 g container of grain alcohol. The silver raises from -100°c to 13°C. Determine the starting temperature of the alcohol. 16) A sample of iron starts at 153°C and cools to 30. °C when dropped in an 810. g container of grain alcohol. The alcohol was originally 3.1°c. What is the mass of the sample of iron? 17) A 128 g gold necklace is dropped into a 422 g vat of mercury. The mercury cools from 99°( to 84°C. What was the original temperature of the gold necklace? 18) A 483 g wood dowel rod is dropped into 1.0 liter of water (1 kg). The temperature of the dowel rod was increased 22°c. The water sample 0 was originally 60. c. What is the new temperature of the water? 19) A gallon of water (3.79 kg) is dumped in 42.3209 kg of chemical X. The water cools from 90°C to 3.0°C. Chemical X was originally at -31.0°(. Calculate the specific heat of chemical X and determine what substance it is. 20) A sample of iron is dropped into 6045 g of chloroform. The chloroform's temperature is raised from 15°( to 57.2°c. If the temperature of the iron block was 81.2°c, determine the mass of the iron in kilograms.