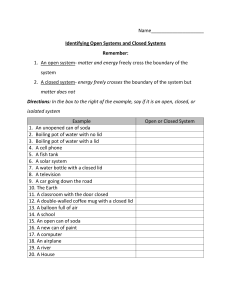
Chemical Bonds Lab Name ____________________ Objectives: 1. To analyze the data and rank the substances according to their melting points. 2. To observe the compounds for their solubility or insolubility. 3. To learn the properties of ionic and covalent compounds. Introduction: Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two types—ionic and covalent. Ionic bonds result when one or more electrons from one atom or group of atoms are transferred to another atom. Positive and negative ions are created. In covalent compounds, the electrons are shared by the bonded atoms. The physical properties of a substance such as melting point, solubility, and conductivity tell us a lot about the type of bond in a compound. Safety: Wear safety goggles and apron. Confine loose clothing and long hair. Equipment: tin can lid conductivity apparatus iron ring and ring stand microtitration plate candle sucrose phenylsalicylate potassium iodide sodium chloride calcium chloride citric acid Procedure: 1. Describe the substance. 2. Obtain a tin can lid and candle 3. attach the iron ring to the ring stand. 4. Place the lid on the ring. 5. Place the candle on wax paper under the lid. The lid should be just above the tip of the flame. 6. Take the lid and inside the rubber ring on the lid, number it from 1-6 being careful to space the numbers evenly from the center of the lid and each other. 7. Place the chemicals on the lid in the following order and do not allow them to touch. ONLY USE VERY FEW CRYSTALS. DO NOT PILE THEM UP. a. citric acid=#1 b. sucrose=#2 c. sodium chloride=#3 d. potassium iodide=#4 e. phenyl salicylate=#5 f. calcium chloride=#6 8. Write the descriptions of the chemicals in the table. 9. Light the candle and be sure it is place on the wax paper. 10. Place the candle under the tin can lid and observe for two minutes. (As the chemicals get hot, they may splatter. Be careful) 11. Record the order of melting. (which melted first is 1, then 2, etc.) After two minutes place an n in the box if the substance did not melt. 12. Remove the candle and wax paper. Gently blow out the flame. 13. Clean up, return the candle, throw wax paper away, throw tin can lid in trash when it cools. 14. Obtain a microtitration place. 15. Place a few crystals of each compound in the top row of the well. 16. Add 10 drops of water to each well. Be careful not to touch the chemicals with the medicine dropper/pipette. 17. Use the stirring rod and stir the water and chemicals in the first well. 18. Observe the well for a minute to determine solubility. Record in the table. 19. Rinse the stirring rod and repeat steps 17-18 for each well. 20. Using the same solutions in the same wells, test for conductivity. 21. Obtain the conductivity apparatus. 22. Dip the electrodes into the first well. If the solution conducts electricity, it will light. Record in the table. 23. Rinse the electrodes and dry them easily after each use. Then proceed to the second well. 24. When finished, dump the contents of the plate into the sink, and wash the plate with water a couple of times. Then obtain a cotton swab and swab each of the wells and rinse. Dry the plate and return. Data Table: Compound Description Melted after 2 min Soluble in water Calcium chloride Citric acid Phenyl salicylate Potassium iodide Sodium chloride Sucrose Conclusion: Based on the observations recorded in the data table, fill in the table with yes or no: Compound Calcium chloride Citric acid Phenyl salicylate Potassium iodide Sodium chloride sucrose Ionic Covalent Conducted electricity