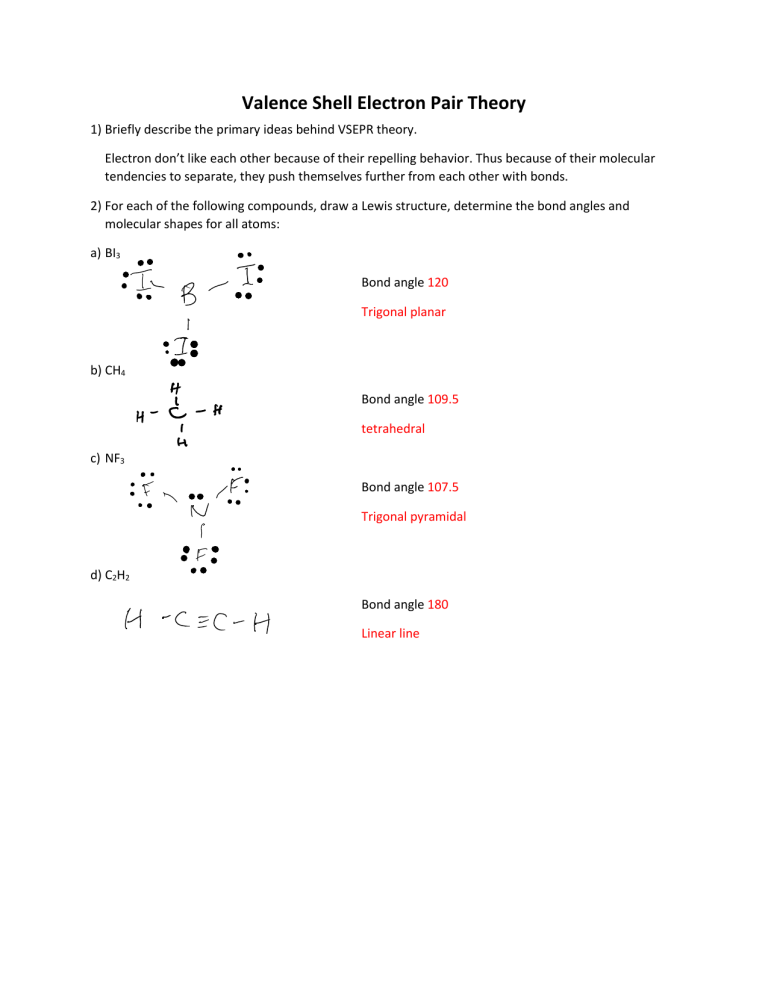
Valence Shell Electron Pair Theory 1) Briefly describe the primary ideas behind VSEPR theory. Electron don’t like each other because of their repelling behavior. Thus because of their molecular tendencies to separate, they push themselves further from each other with bonds. 2) For each of the following compounds, draw a Lewis structure, determine the bond angles and molecular shapes for all atoms: a) BI3 Bond angle 120 Trigonal planar b) CH4 Bond angle 109.5 tetrahedral c) NF3 Bond angle 107.5 Trigonal pyramidal d) C2H2 Bond angle 180 Linear line



