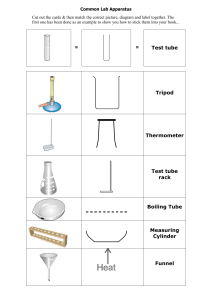
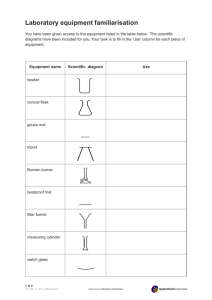
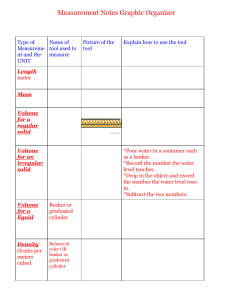
Law Lab Report Tuesday, January 26, 2020 All experiments begin with a hypothesis that answers the question, "What influence does the temperature of a gas have on its volume?" "If a certain amount of gas is heated, the volume will rise because the heat will cause the gas molecules to move quicker and farther apart," I hypothesize. I'd have to adjust the temperature of the gas between 0 and 100 degrees Celsius to test this idea. The temperature and volume are the variables in this experiment. The volume is a dependent variable, while the temperature is an independent variable. A 600 mL beaker, tongs, tweezers, a thermometer, a centimeter ruler, a wire gauge, a hot plate, heated pad, ice, a rubberband, capillary tube, and water are included in the kit. To begin the experiment, I take a 0.2cm radius measurement of the capillary tube. Then I begin to prepare. I take a 0.2cm radius measurement of the capillary tube. Then I start prepping the tube by inserting an oil stopper and using the rubberband to attach the capillary tube to the centimeter ruler. After that, I fill the beaker with ice and water until it reaches 600 mL, then place the tube and ruler inside. The volume of air is then measured around 0 degrees Celsius. To place the beaker on the hot plate, I use tongs. When the water is hot enough, I remove the beaker with the tong and begin measuring the gas's details. For instance, suppose the temperature is 3°C, the volume is 0.72cm, and the height is 5.7cm3. The rest of my experiment is then repeated in similar manner. I'm currently measuring the volume of air at a temperature of around 20 degrees Celsius. The temperature has reached 21 degrees. 313 0.84 3 3 0 0.8 7 3 5 5 0.9 3