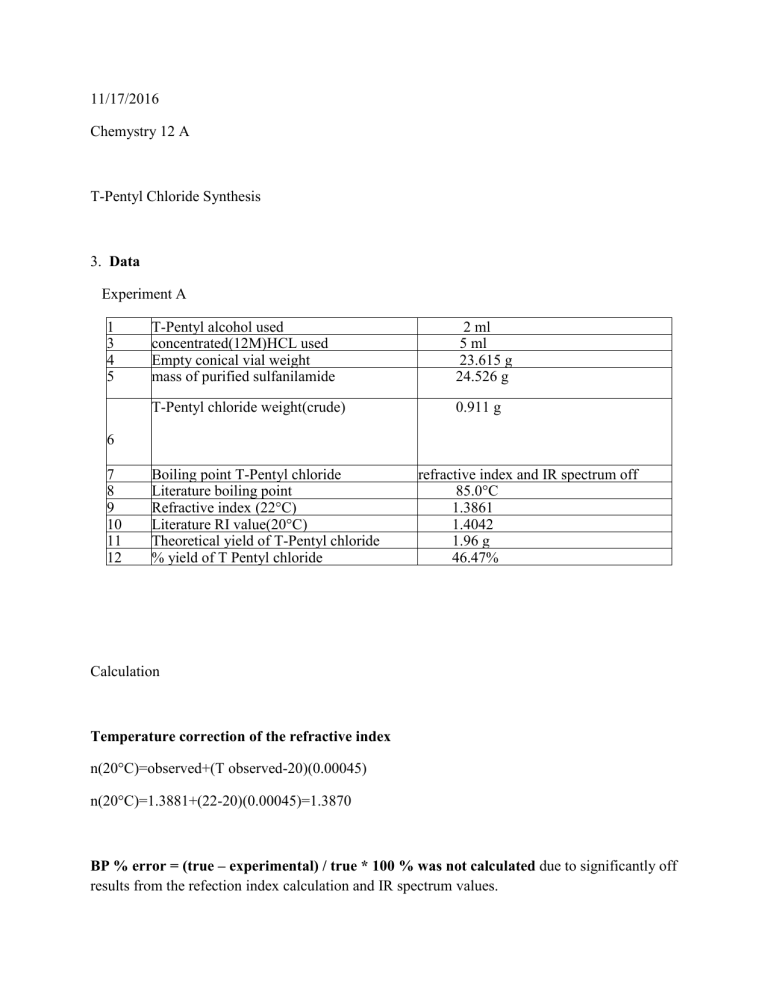
11/17/2016 Chemystry 12 A T-Pentyl Chloride Synthesis 3. Data Experiment A 1 3 4 5 T-Pentyl alcohol used concentrated(12M)HCL used Empty conical vial weight mass of purified sulfanilamide 2 ml 5 ml 23.615 g 24.526 g T-Pentyl chloride weight(crude) 0.911 g 6 7 8 9 10 11 12 Boiling point T-Pentyl chloride Literature boiling point Dsssssssssssssssssssssulfanilamide Refractive index (22°C) Literature RI value(20°C) Theoretical yield of T-Pentyl chloride % yield of T Pentyl chloride refractive index and IR spectrum off 85.0°C vvvvvalues 1.3861 1.4042 1.96 g 46.47% Calculation Temperature correction of the refractive index n(20°C)=observed+(T observed-20)(0.00045) n(20°C)=1.3881+(22-20)(0.00045)=1.3870 BP % error = (true – experimental) / true * 100 % was not calculated due to significantly off results from the refection index calculation and IR spectrum values. Limiting reagent calculations Moles T-Pentyl alcohol used 2ml*(0.8096 g/ml)*(1mol/88.15g) =0.0184 mol alcohol Moles HCl used 5ml*(1.18g/ml)*(1mol/36.47g) =0.162 mol HCL Alcohol is limiting agent Moles of alcohol 1:1 ratio alcohol/T-Pentyl chloride=moles T pentyl chloride 0.0184mol *1:1 =0.0184 mol T-Pentyl chloride Theoretical yield =0.0184g T-Pentyl chloride*(106.6g/mol) =1.96g T pentyl chloride % yield= actual yield/theoretical yield *100% % yield=0.911g/1.96g*100%=46.47% Conclusion The goal of the experiment is to synthetize T pentyl chloride from T-Pentyl alcohol and HCl via SN1 reaction . T pentyl alcohol reacts that with hydrochloric acid. In the first step of the reaction, the lone pair of electrons from oxygen are going to go and abstract a proton. The electrons between the hydrogen and chlorine bond are going to end up on the chlorine giving Cl minus and also we get bound water structure as an intermediate .In step 2, that water molecules going to leave and the electrons between the carbon-oxygen bonds are going to go onto the oxygen and that will give us a carbo cation intermediate and water. As soon as the carbo cation intermediate form, in step 3, the chlorine anion that we formed in step one is going to go and attack that positive charge. Positive things are attracted to negative things and we end up with t-Pentyl Chloride as our product. Overall my experiment was a failure. When I used refraction index value and IR spectrum to characterize the purity of the products, the results were significantly off. Literature RI value (20°C) for t-Pentyl Chloride is 1.4042 .My result was 1.3861.IR spectrum of t-Pentyl Chloride has a C-H peak at 2900 cm-1 and C-Cl bending peaks at 800 cm-1.My result resemble alcohol impurity with lower frequencies and O-H broad peak at 3300 cm-1.The main difference is O-H peak. Possible sources of errors are probably due to 1. I did not completely removed the bottom layer 2. and or washed properly with water and Na2SO4 quickly the top layer(T-Pentyl chloride) and neutralized the remaining acid .Acid probably catalyzed reversed reaction (hydrolyzes). 3. Mistakes in the procedure due to inexperience and miscalculating the timing A pure substance generally has a boiling range of one or two degrees. Boiling range is the difference between the temperature where the compound starts to boil and the temperature where boiling is complete of one or two degrees. When you compare boiling point ranges the most important thing is to observe if your boiling point range is at the literature range for the pure substance or is below that range. If it is below the range it means that the compound is impure. I did not do the boiling point of T-Pentyl chloride or calculated BP % error = (true – experimental) / true * 100 %, because my refraction index and R spectrum of the product was significantly off. These results suggest that the product is not pure compared to T-Pentyl chloride because its boiling point range and the boiling point itself would not be close to the actual boiling point range and the boiling point of pure T-Pentyl chloride.





