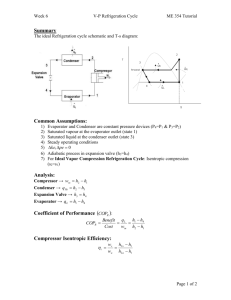
DEPARTMENT OF MECHANICAL AND INDUSTRIAL ENGINEERING FACULTY OF ENGINEERING AND BUILT ENVIRONMENT Refrigeration Bench Experiment SURNAME : NOPAPAZA INITIALS : A.C STUDENT NO : 217033248 COURSE : Refrigeration and Air Conditioning 3B MODULE : RACMIB3 DATE : 2021/11/03 I confirm that this assignment is my work, is not copied from any other person's work, and has not previously submitted for assessment either at the University of Johannesburg or elsewhere. Signed: NOPAPAZA AC. Date: 14 Oct. 20 Table of Contents Introduction ................................................................................................................................ 2 Comparing Vapour Compression Systems to Air Refrigeration Systems ............................. 3 Advantages of VCRS over Air Refrigeration Systems ................................................. 4 Disadvantages of VCRS over Air Refrigeration Systems ............................................ 4 Classification of Evaporators ................................................................................................. 4 Aim ............................................................................................................................................ 7 Apparatus and Materials ............................................................................................................ 8 Assumptions............................................................................................................................... 8 Observations .............................................................................................................................. 8 Analysis of Results .................................................................................................................... 9 Observation 1 ......................................................................................................................... 9 Properties of R22 at 3.13 bar .......................................................................................... 9 Observation 2 ....................................................................................................................... 11 P-H diagram of the system ................................................................................................... 12 Discussion ................................................................................................................................ 13 Recommendations ................................................................................................................ 14 Conclusion ............................................................................................................................... 14 Bibliography ............................................................................................................................ 15 Appendix .................................................................................................................................. 16 1 Figure 1: Schematic of Vapour Compression Home Refrigeration System [1] ........................ 3 Figure 2: Lab Vapour Refrigeration System .............................................................................. 8 Figure 3: Pressure Enthalpy Diagram for Superheated Vapour at Evaporator Exit. ............... 12 Table 1: Observation table ......................................................................................................... 8 Table 2:Interpolation to find properties at 3.13 bar ................................................................... 9 Table 3:Interpolation to find exact values of point 1 ............................................................... 10 Table 4: Interpolation to find point 2 properties at 5.9bar ....................................................... 10 Table 5: Interpolation to find the exact values at point 2 ........................................................ 10 Table 6: Point 3 values reading ................................................................................................ 11 Table 7:Summary of data points (Observation 1) .................................................................... 11 Table 8: Summary of data points (Observation 2) ................................................................... 12 Figure_AX 1: Observation 1 Calculations ............................................................................... 16 Figure_AX 2:Observation 2 Calculations ................................................................................ 17 2 Introduction Refrigeration systems are ubiquitous in modern life. They are used in food preservation, manufacturing and processing. Refrigeration systems are also found in industrial and commercial applications where they are needed to keep an environment at a constant temperature. There are different types of refrigeration systems and methods of transferring heat. Vapour compression systems use the condensation and vaporisation of substances called refrigerants that have low boiling points and can be condensed in a wide range of pressures and temperatures. Figure 1: Schematic of Vapour Compression Home Refrigeration System [1] Comparing Vapour Compression Systems to Air Refrigeration Systems Vapour compression systems are commonly used for all air conditioning and air conditioning systems. They are said to be an improvement in refrigeration from gas cycle systems. The first distinction of vapour compression systems from gas cycle refrigeration systems is their 3 working principle. Gas cycle refrigeration systems use sensible heating and cooling to extract heat and refrigerate the warm region. Vapour compression refrigeration systems use latent heat to refrigerate the warm space as the refrigerant undergoes phase change during vaporisation. Some of the other distinctions between the VCRS and air refrigeration systems are discussed in the form of the advantages and disadvantages VCRS have compared to air refrigeration systems: Advantages of VCRS over Air Refrigeration Systems VCRS have a high refrigeration capacity while requiring small mass flow rates. This means VCRS can be much smaller than air refrigeration systems for the same output. Air refrigeration cycles require significant modifications to be made to the system to obtain maximum output. In contrast, VCRS require less equipment for the improvement of performance comparable to the air refrigeration systems, hence they have less operational costs [1]. Vapour compression refrigeration systems have a higher coefficient of performance than air refrigeration systems. This means that they produce a higher refrigeration effect with less work input required. Due to the very low boiling points of the refrigerants and wideband of condensation temperatures, VCRS can be used in a wide range of temperatures, which is increased versatility [2]. Disadvantages of VCRS over Air Refrigeration Systems The substances (Ammonia, Carbon Dioxide, Chlorofluorocarbons) used in VCRS can be harmful to humans, animals and the environment when out in the atmosphere. VCRS require a significant amount of attention and resources to prevent leakages [1]. In large industrial applications, the equipment needed to complete the vapour compression cycle (evaporators, compressors, condensers and expansion systems) can be an expensive initial cost [1]. As mentioned, the substances used in VCRS are dangerous to the environment and may be subjected to supply bans and limitations affecting ease of use. Classification of Evaporators Evaporators are the devices that are responsible for the refrigerating effect or the removal of heat from the system to the environment. This is done by maintaining the refrigerant at a 4 temperature that is lower than that of the area intended to be cooled. From the second law of thermodynamics, the heat is removed from the area and carried by the refrigerant, which evaporates, hence the naming of the equipment as evaporators. There are different kinds of evaporators and their classification can be a function of their construction, method of feeding the refrigerant, the type of heat transfer and their operating conditions. The different types of evaporators are detailed and summarized as follows: Construction 1. Bare tube coil evaporator – These are also known as prime-surface evaporators. Bare tube coil evaporators feature a bare tube that is constructed in a way to allow for vaporisation of the refrigerant. Critical parameters that affect the performance of a bare time coil evaporator include the length of the tube, which increases the surface area of heat transfer, the capacity of the expansion valve – the expansion valve needs to be suited to the length of the evaporator, the diameter of the tube relative to tube length – this should be sized to allow a sufficient flow velocity for vaporisation [2]. 2. Finned tube evaporator – This is the most common type of heat exchanger used in the interface between air and the refrigerant. Fins are attached to the tubes carrying the refrigerant, the effectively increase the contact surfaces for heat transfer. Finned tube evaporators are used in low-temperature applications slightly above (0°C). Finned tube evaporators are prone to frost which can clog airflow and affect the performance of the refrigerator. The length of the tubes is to be controlled to prevent high-pressure drop across the evaporator [2]. 3. Plate evaporator – As it has been established that increasing the heat transfer contact surface increases the performance of evaporators, plate evaporators achieve this by having a plate welded to one or both sides of the evaporator tubes [2]. 4. Shell and tube evaporator – The construction consists of a shell vessel which has the liquid that is to be chilled and several horizontal tubes that house the refrigerant. As the liquid refrigerant is moved through the network of horizontal pipes it exchanges heat with the warmer water or brine solution that has to be chilled. The water or brine solution goes through inlet and outlet headers with perforated metal tube sheets. Shell and tube evaporators can be operated as dry expansion evaporators, with the refrigerant housed in the tube and as flooded evaporators, with the refrigerant circulating in the shell, the later increases refrigeration capacity [2]. 5 5. Shell and coil evaporators - These are similar to shell and tube evaporators in their construction and use of cooling water but differ in that they use coils to circulate the refrigerants [2]. 6. Tube-in-tube evaporators – These evaporators have the same working principle as coaxial heat exchangers, as one tube is nested in another. They provide higher heat transfer but have the drawback of requiring more space for the same refrigerating capacity as the other types of evaporators. They are used in petrochemical and beverages industries [2]. Feeding Mechanism 1. Flooded evaporator – In these refrigeration systems, a surge tank is used to maintain a constant level of liquid refrigerant in the evaporator. The liquid is constantly vaporised in the evaporator and the level of the fluid drops, the surge tanks makes up the drop in the liquid. The refrigerant level in the accumulator drops and is sensed by a float level which is linked to a float valve that opens and lets in liquid refrigerant from the receiver until the float level returns to the required level. Higher heat transfer is achieved through the constant contact of the evaporator coil with the liquid refrigerant. Flooded evaporators are used in the chemical and food processing industries [2]. 2. Dry expansion evaporator – In contrast to flooded refrigeration systems, dry expansion systems have the flow of liquid refrigerant regulated by the expansion valve. This configuration allows for the vaporisation of liquid refrigerant to occur with less space and less refrigerant volume. Dry expansion evaporators are well suited for compact refrigeration systems. The flow of the refrigerant is in one direction and the best efficiency is achieved when the liquid and vapour in the coil is separated with the liquid preferably located at the bottom of the coil and the gas located at the top of the evaporator coil. The flow regulation of the expansion valve and the diameter of the coil should be configured to reduce the chances of bubbles developing in the system that reduce the heat transfer of the system [2]. Heat transfer mode 1. Natural convection evaporators – The flow of air in these types of evaporators is guided by the natural movement of air. They use the fundamental guiding principle that warm air rises and cold air descends. An example of this is the high vertical placement 6 of evaporators in domestic refrigeration to allow the cold air to descend on to the refrigeration chamber [2]. 2. Forced convection evaporator – The movement of air over the refrigerant is pushed through a fan driven by an electric motor. This is a more efficient method of heat transfer as less surface area is required and load on the compressor can be achieved through increasing evaporator pressures. Evaporator operation conditions This refers to the temperature operating conditions that the evaporator is operating in. As mentioned, VCRS have a wide band of operating temperatures and they can be outlined as follows [2]: 1. Frosting evaporator – These evaporators operate below the freezing point of water (0°C). This caused moisture from the atmosphere to freeze on the surface of the evaporator and cause frost. This frost has to be removed manually or automatically as the build-up of frost negatively impacts the performance of the refrigerator. 2. Non-frosting evaporator – They operate above but close to (0°C). Therefore, frosting does not occur. This application is suited for high-temperature applications where shrinkage and dilution due to frosting are avoided as in bakery refrigeration. 3. Defrosting evaporator – They use the on and off cycles of evaporators to manage to frost. During the on-cycle of the compressor, frosting occurs and defrosting take place during the off-cycle of the compressor. This is done through rapid heat transfer through the type of evaporator construction [2]. Aim The experiment aims to familiarize the student with vapour compression systems and the use of pressure-enthalpy diagrams and property tables to determine the system Coefficient of Performance (COP) 7 Apparatus and Materials Figure 2: Lab Vapour Refrigeration System Assumptions The following assumptions were made for the experiment: The system had no leaks. The evaporator and condenser pressures stayed constant throughout. There was no undercooling at the condenser exit, i.e. saturated liquid refrigerant. Observations The following readings were observed for the vapour compression refrigeration system: Table 1: Observation table Description Evaporator Pressure Condenser Pressure Evaporator Outlet Temperature Symbol Pe Pc t1 Units kPa kPa °C Observation 1 313 590 -3 Observation 2 335 590 -4 8 Analysis of Results The observation results from the laboratory experiment were used to find the enthalpies, temperatures and the performance characteristics of the laboratory refrigeration system. The detailed calculations with detailed explanations of the methodology are attached in the Appendix. The following was established about the system: The refrigerant at the evaporator exit is superheated. The refrigerant at the condenser exit is not supercooled. The values that were obtained from the laboratory were not present as exact values in the R22 property table. Interpolation was employed to find the properties of the refrigerant at the various points in the system. A summary of the analysis results is presented. To aid in concise reading, the detailed steps followed for the second set of results are attached in the Appendix. Observation 1 Properties of R22 at 3.13 bar Table 2:Interpolation to find properties at 3.13 bar Tsat [°C] Pressure h[kJ/kg] s[kJ/kg] h[kJ/kg] s[kJ/kg] [bar] [ΔTsup = 10K] [ΔTsup = 10K] [ΔTsup = 20K] [ΔTsup = 20K] -15 2.9570 406.24 1.8009 412.97 1.8255 -13.5 3.13 406.88 1.7985 413.65 1.8201 -10 3.5430 408.41 1.7927 415.26 1.8174 From the temperature at evaporator exit, we see that the vapour is superheated as the temperature at exit (-3°C) is higher than the saturation temperature (-13.5°C). We then find the degree of superheat: 9 Δ𝑇 = 𝑡𝑠𝑢𝑝 − 𝑡𝑠𝑎𝑡 = −3 − (−13.5) = 10.5𝐾 It was observed that the exact values for point 1 lie between 10K and 20K degree of superheat, therefore, linear interpolation is to be done in between these values: Table 3:Interpolation to find exact values of point 1 T [°C] P[bar] h[kJ/kg] s[kJ/kgK] -13.5+10=-3.5 3.13 406.88 1.7985 -13.5+10.5= -3 407.22 1.7996 -13.5+20 = 6.5 413.65 1.8201 𝑘𝐽 𝑘𝐽 Therefore, at evaporator exit, the properties are ℎ1 = 407.22 𝑘𝑔 , 𝑠1 = 1.7996 𝑘𝑔 , 𝑡1 = −3°𝐶. 𝑘𝐽 As we assumed isentropic compression ∴ 𝑠1 = 𝑠2 = 1.7996 𝑘𝑔, we then use this entropy value to find the temperature of the gas and other properties at compressor pressure (590kPa) through linear interpolation: Table 4: Interpolation to find point 2 properties at 5.9bar T [°C] P[bar] s[kJ/kgK] [Δ𝑇 = 20𝐾] s[kJ/kgK] [Δ𝑇 = 30𝐾] h[kJ/kg] h[kJ/kg] [Δ𝑇 = 20𝐾] [Δ𝑇 = 30𝐾] 5 5.8378 1.7956 1.8200 421.02 429.23 5.32 5.9 1.7952 1.8196 422.02 429.37 10 6.8078 1.7894 1.8137 423.97 431.47 It is seen that point 2 (compressor exit) lies between the 20 and 30K superheat. We again use interpolation to get the temperature of the refrigerant after compression and enthalpy. Table 5: Interpolation to find the exact values at point 2 T [°C] P[bar] s[kJ/kgK] h[kJ/kg] 5.32+20=25.32 5.9 1.7952 422.02 27.12 1.7996 423.335 5.32+30 = 35.32 1.8196 429.37 10 Finding point 3, after the condenser where the refrigerant is in a liquid phase. Enthalpies are found through the interpolation table. Table 6: Point 3 values reading T [°C] 5 5.32 10 P[bar] 5.8378 5.9 6.8078 hf [kJ/kg] 205.9 206.28 211.88 The bolded characters in the table are the properties of the saturated liquid at compressor exit. Since the fluid goes through an expansion valve, the enthalpy remains constant, h3 = h4 = 206.28 kJ/kg. The table of properties at the various points in the refrigeration system is presented as follows: Table 7:Summary of data points (Observation 1) Point 1 2 3 4 Temperature -3 27.12 5.32 H[kJ/kg] 407.22 423.35 206.28 206.28 S[kJ/kgK] 1.7996 1.7996 The coefficient of performance is then given as: 𝐶𝑂𝑃 = = ℎ1 − ℎ4 ℎ2 − ℎ1 407.2 − 206.8 423.35 − 407.22 = 12.43 Observation 2 As previously mentioned, the detailed analysis of the second set of results is outlined in the Appendix section. A few salient points about this process were summarized below and the table of results is provided: At the exit of the evaporator, the degree of superheat was found to be = -4°C – (-11.70 °C) = 7.7K. At the compressor exit, the exact point 2 lies in between the 10K and 20K degree of superheat values. 11 Table 8: Summary of data points (Observation 2) Point Temperature[°C] H[kJ/kg] S[kJ/kgK] 1 -4 406.126 1.7996 2 22.94 420.25 1.7996 3 5.32 206.28 4 206.28 The coefficient of performance is given as: 𝐶𝑂𝑃 = = ℎ1 − ℎ4 ℎ2 − ℎ1 406.126 − 206.28 420.25 − 406.126 = 14.15 P-H diagram of the system Figure 3: Pressure Enthalpy Diagram for Superheated Vapour at Evaporator Exit. The pressure-enthalpy of a refrigeration system with superheated vapor at evaporator exit in Figure 3 12 Discussion The vapour compression refrigeration system was observed in practice and the parameters of the system were noted from the different gauges in the apparatus. The following salient points were noted about the experiment: The temperatures at evaporator exit revealed some information about the modification to the basic vapour compression cycle. The refrigerant was superheated at the exit of the evaporator. This was established through the calculation of the saturation temperature of the refrigerant at the given pressure. For the first set of observations, the degree of superheat from the saturation temperature (-13.5 °C) was Δ𝑇 = 10.5𝐾 and from the second set of readings, the degree of superheat from the saturation temperature (-11.70 °C) was Δ𝑇 = 7.7𝐾. From the table of observations, it was noted that the evaporator pressure was raised. The effect of this modification is discussed later. The laboratory technician outlined another parameter that was changed and that is the rate of cooling. For the first set of readings, the rate of cooling was reported to be 40 and for the second set of readings, the rate of cooling was reported to be 60. From the analysis of results, it could be seen that the second set of values yielded a higher coefficient of performance 14.15 compared to 12.43. The increase in the coefficient of performance could be due to the increase in evaporator pressure. This agrees with the theory from the literature that the lowering of the evaporator pressure decreases the performance of the refrigerator. The effect of the evaporator pressure can be observed in the COP formula. The compression process 1-2 is the denominator in the COP formula, therefore it is inversely proportional to COP, i.e. an increase in the work done by the compressor decreases the performance of the refrigeration system. The second set of readings has a lower refrigeration capacity but has a lower work input, hence improving the performance. Superheating at both the evaporator and compressor exit improves the performance of the refrigerator, this is however limited by how low the evaporator pressure and temperature is. 13 The rate of cooling is a function of different parameters namely, volumetric fluid flow, surface area, thermal conductivity and the temperature difference between the refrigerant and the environment [3]. The mechanism of increasing the cooling rate in the refrigerator was not outlined to the group of students. Recommendations Only a single recommendation was humbly made by the student upon reflection on the lab: A walk-around, by the technician, for the different knobs and buttons could be helpful. Conclusion In conclusion, the operation of a vapour refrigeration system was appreciated practically and the parameters that were used in theory calculations were read from the physical machine. Thus, part of the aim of the experiment was met. Pressure enthalpy (p-h) graphs were used to gain an understanding of the cycle in the graphs and the changes that come about modifying different parameters. Property tables and interpolation was used to find the values that were not present in the standard R22 refrigerant table. The concept of the rate of cooling was introduced as a performance characteristic and was found to be affected by parameters such as flow rate and surface area. It was observed that the increase in the evaporator pressure increases the coefficient of performance (COP) as it decreased the amount of work required from the compressor. Finally, with all the observations, learnings and discussion about the operating of a vapour compression system, its comparative nature against air refrigeration systems and the different types of evaporators, the aim of the experiment was successfully met. 14 Bibliography [1] J. Tomczyk, E. Silberstein, B. Whitman and B. Johnson, “Refrigeration,” in Refrigeration and Air Conditioning Technology, Boston, Cengage Learning, 2016, pp. 498-520. [2] R. Khurmi and J. Gupta, A Textbook of Refrigeration and Air Conditioning (SI Units), New Dehli: Eurasia Publishing House (P) Ltd. , 2009. [3] Posthavest Management of Vegetables, “Cooling Rates,” Posthavest Management of Vegetables, Sydney. 15 Appendix Figure_AX 1: Observation 1 Calculations 16 Figure_AX 2:Observation 2 Calculations 17