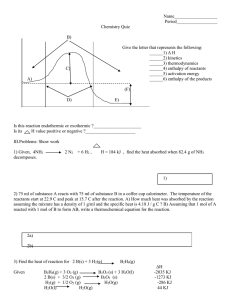
TRENDS :-Arrange the following Q3 in increasing order of given property [Bond angle of ONO bonds] Ans -ANS- NO2- < NO2< NO2+ Q2 Q4 i O2 F2, O F2, Cl2O Br2O [thermal stability] ii Cl2O, ClO2, Cl2O6, Cl2O7 [thermal stability] iii H2O, O F2, Cl2O Br2O [bond angle] iv H2O,HF,NH3,NF3 [ dipolemoment] ANS- v vi bond strength bond strength vii bond length Ans i O2 F2< O F2<Cl2O< Br2O iiii , v ii Cl2O, <ClO2< Cl2O6<Cl2O7 O F2<H2O <Cl2O <Br2O [bond angle ivi vi vii 10 REASONING BASED QUESTIONS FROM P-BLOCK ELEMENTS 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 GROUP-15 Nitrogen does not form pentahalide although it exhibit +5 oxidation state. Due to absence d-orbitals N can not extend its valency beyond four PH3 has lower B.pt than NH3 N is more electronegative than P so in NH3 there is intermolecular H-bonding hence it has high b.pt NH3 acts as Lewis base Because N has a lone pair electron so NH3 acts as a Lewis base NO2 dimerises NO2 has an odd electron so it dimerises to pair up electron and to achieve octet configuration NH3 is stronger base than PH3 Due to smaller size of nitrogen there is high electron density on nitrogen so electron pair is easily available. PCl3 fumes in moisture PCl3 undergoes hydrolysis and gives fumes of HCl. PCl3 + 3 H2O H3PO3 + 3HCl All the five P-Cl bonds are not equal in PCl5 The two axial bonds suffer more repulsion from equatorial bonds and hence are elongated. H3PO2 has reducing character Since it has two P-H bonds H3PO3 is dibasic (diprotic) but H3PO4 is tribasic In H3PO3 only two H atoms are linked to O which are ionisable the third H is attached to P and not ionisable because P is less electronegative. In H3PO4 all the three H atoms are with O and ionisable PCl5 is ionic in solid state It is due to the following conversion : 2PCl5 [PCl4]+[PCl6]NO is paramagnetic in gaseous state but diamagnetic in liquid and solid state NO(g) has odd number of electrons so it is paramagnetic but in liquid and solid state it exists as dimmer so there is no unpaired electron and it will be diamagnetic NCl3 hydrolysed but NF3 does not In NCl3 Cl has vacant d-orbitals to accept the lone pair from H2O but in NF3 F has no d-orbitals NCl3 + 3H2O NH3 + 3 HOCl Nitrogen shows little catenation but phosphorous distinctly shows catenation property Due to smaller size of N there is repulsion between the lone pairs and N-N single bond is weaker than P-P +5 oxidation state of Bi is less stable than +3 Because inert pair effect is very prominent in Bi , so +5 oxidation state is not stable Bi in +5 oxidation state is strong oxidizing agent Because inert pair effect is very prominent in Bi so Bi5+ can be easily converted into Bi3+ NO(nitric oxide) becomes brown when released to air It oxidizes to NO2 NH3 is a good complexing agent/ NH3 acts as a ligand It has lone pair of electron on N-atom and can be donated for the coordination bond. Bi2O3 is not acidic The size of Bi3+ is very large and so there is very weak +ve electric field around it so it does not interact with water to release H+ BiH3 is the strongest reducing agent among the group-15 hydrides Since Bi-H bond is the weakest among pr-15 hydrides so H2 gas is evolved which is reducing N2 is less reactive at room temperature Due to having triple bond and hence high bond dissociation energy(946 kJ/mol) Bond angle in PH4+ higher than in PH3 In PH3 there is lp-bp repulsion so bond angle is less where as in PH4+ there is no lp-bp repulsion 1 22 NH3 has greater bond angle than PH3 N is more electronegative so it attracts the bond pair electron and hence there is greater bp-bp repulsion in NH3 and hence greater bond angle 23 R3P=O exists but R3N=O does not N due to absence of d-orbitals can not form pπ-dπ multiple bond 24 N exists as N2 but P exists as P4 Due to smaller size N can form pπ-dπ multiple bonding and exists as discrete N2 molecule but P can not form pπ-pπ multiple bonding. 25 PCl5 can not act as reducing agent In PCl5 P has +5 oxidation state. P has five valence electron in its valence shell so it can not increase its oxidation state beyond +5, so it can not act as reducing agent. 26 Phosphorous is kept under kerosene It is highly reactive and easily catches fire due to air oxidation 27 H3PO3 is syrupy liquid Due to intermolecular H-bonding 28 PH3 bubbles but NH3 dissolves in water NH3 forms H-bonding with water but PH3 can not form so NH3 dissolves but PH3 bubbles out 29 Only a small increase in radius is observed from As to Bi Due to poor shielding effect of d and f obitals. 30 Nitrogen is gas where as phosphorous is solid at room temp. Nitrogen is diatomic molecule having weak van der Walls attraction where as phosphorous is tetra atomic so it has strong van der Walls attraction. 31 N-N bond is weaker than P-P bond Due to shorter bond length there is greater repulsion between lone pairs in N2 32 Maximum number of covalent bond formed by N is four Because it has three unpaired electrons and one lone pair. 33 P2O5 can not be used for drying ammonia gas. P2O5 is acidic it reacts with ammonia in presence of moisture and form (NH4)3PO4 34 NO2 is coloured but its dimmer N2O4 is colourless Because NO2 has unpaired electron so it can absorb light from VR 35 Acidity of oxyacids of nitrogen increases with increase in oxidation number of N Because non metallic character increases with oxidation number 36 White phosphorous is more reactive than red phosphorous White phosphorous consists of discrete P4 molecules which is tetrahedral so reactive but in red phosphorous the P4 molecules are linked in extended chain structure so it is less reactive. 37 Phophinic acid ( H3PO4) is mono basic / mono protic Only one H atom is linked with O which is ionisable 38 N2 has higher bond dissociation energy than NO Because N2 has higher bond order 39 N2 and CO have same bond order but CO is more reactive CO is polar molecule 40 (CH3)3N is pyramidal but (SiH3)3N is planar (CH3)3N is pyramidal due to sp3 hybridisation and one lone pair on N but (SiH3)3N is planar due to sp2 hybridisation and its lone pair is donated to vacant d orbital of Si for pπ-dπ overlap 41 The first IE of N is greater than that of O It is due to half filled and hence stable electronic configuration of N 42 HNO2 disproportionates In HNO2 the N is in +3 oxidation state which may increase as well as decrease 43 PCl5 can not act as reducing agent In PCl5 phosphorous is in +5 oxidation state that is the highest oxidation state of P. 2 GROUP-16 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Group 16 elements have lower I.E with compare to corresponding group 15 elements Because group 15 elements have stable half filled p-sub shell(ns2np3) so electron can not be removed easily H2S is less acidic than H2Te In H2Te there is lower bond energy of H-Te bond due to larger size of Te H2S acts as reducing agent while SO2 acts as both In H2S , S has its minimum oxidation state -2 where as in SO2 it is +4 so it can be decreased up to -2 or increased up to +6 , So H2S is only reducing but SO2 is both. H2S is acidic while H2O is neutral H-S bond is weaker due to larger size of S so proton release easier in H2S SF6 is known but SH6 does not exist Fluorine is the strongest oxidizing agent so it can oxidizes S to its maximum oxidation state +6 , H can not Compound of F & O is fluoride of oxygen not oxide of fluorine F is more electronegative than O SCl6 is not known but SF6 is known F is strongest oxidizing agent so it can oxidizes S to its maximum oxidation state +6 . Cl can not. Again Cl has larger size so steric repulsion is there in SCl6 SF6 is used as gaseous electrical insulator It is thermally stable and chemically inert SF6 is not easily hydrolyzed It is sterically protected by six F atoms hence does not allow H2O molecules to attack the S atom S exhibits catenation properties but not Se Due to smaller size of S than Se. S-S bond is much stronger than Se-Se bond S disappears when boiled with Na2SO3 It forms sodium thiosulphate . Na2SO3 + S Na2S2O3 ( soluble) H2O is liquid but H2S is gas O is electronegative so there is intermolecular H-bonding in water so it is liquid. Ozone is powerful oxidizing agent It decomposes to form nascent oxygen Ka2 is less than Ka1 , for H2SO4 in water The 2nd proton releases from HSO4- which is difficult. So Ka2 is less than Ka1 O2 is gas but sulphur is solid Due to smaller size O can form pπ-pπ multiple bond and exists as discrete diatomic molecule. Group 16 elements are called chalcogens Chalcogen means ore forming elements. They form several ores Positive oxidation states of O are generally not found Due to high electro negativity of O Thermal stability decreases from H2O to H2Te in group 16` Due to increase in atomic size from O to Te the bond dissociation energy decreases O does not show +4 & +6 oxidation states like S Due to absence of d-orbital in oxygen The magnitude of electron gain enthalpy of oxygen is less than that of sulphur Due to very small size of O there is inter electronic repulsion Among the hydrides of group 16 water shows unusual properties Due to H-bonding in water the molecules get associated S exhibits catenation but not O Because S-S bond is stronger than O-O bond 3 23 Tendency to show -2 oxidation state diminishes from O to Po in group 16 Due to decreases in electronegetivity moving down the group 24 O2 is paramagnetic although it has even number of electrons Due presence of unpaired electrons in anti bonding molecular orbitals 25 Sulphur in vapour state exhibit paramagnetism In vapour stare sulphur partly exists as S2 molecule and like O2 it has unpaired electrons in π* orbitals 26 SF6 is less reactive than SF4 In SF6 sulphur atom is sterically hindered due to six F atoms GROUP-17 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Halogens have maximum negative electron gain enthalpy(∆egH) Because they have smallest size in their respective periods F has less electron gain enthalpy than that of Cl but fluorine is stronger oxidizing agent than chlorine F has very small size so there is interelectronic repulsion. F is stronger oxidizing agent due to its low bond dissociation energy and high hydration energy F exhibits only -1 oxidation state , other halogen shows +1, +3, +5, +7 oxidation states F is most electronegative element and due to absence of d-orbitals it can not expand its octet so it does not exhibit positive oxidation state. Iron reacts with HCl gives Fe(II)chloride and not Fe(III)chloride Fe + 2 HCl FeCl2 + H2 H2 liberated prevents the oxidation of FeCl2 to FeCl3 Bond dissociation energy of F2 is less than Cl2 Due to very small size of F there is interelectronic repulsion in F2 so it has low bond dissociation energy Fluorine does not undergo disproportionation Disproportination means simultaneous oxidation-reduction. F being the most electronegative element undergoes only reduction but not oxidation. NO dimerises but Cl2O does not NO is odd electron species so it complete its octet by dimerisation Bleaching by Cl2 is permanent but by SO2 is temporary Cl2 bleaches by oxidation while SO2 does it by reduction. The reduced product gets oxidized again in air and the colour returns HF has lower acid strength than HI Due to larger size of I , the H-I bond is weaker than H-F bond so HI is stronger I2 is more soluble in KI than in water I2 forms complex with KI i.e K+I3HClO is stronger acid than HIO ClO- is more stable than IO- because Cl is more electronegative, so HClO is stronger HClO4 is stronger acid than HClO3 ClO4- is more stable than ClO3- due to more resonance OF2 should be called fluoride of oxygen and not oxide of fluorine Because F is more electronegative than O Interhalogens are more reactive than halogens They are polar HF is stored in wax coated glass bottle HF reacts with alkali present in glass. MF is more ionic than MCl ( M is alkali metal) Because F- is smaller than Cl- and hence it is less polarisable. Cl2 + KI brown, but excess Cl2 turns it colourless 18 HClO4 is stronger than H2SO4 4 19 20 21 22 23 24 25 26 27 28 Because the conjugate base ClO4- is stable due to resonance ClF3 exists but FCl3 does not F is smaller in size and can not accommodate three chloride ions due to steric factor. HF is less volatile than HCl In HF there is intermolecular H-bonding so the HF molecules get associated F form only one oxo acid , HOF Due to absence of d-orbital it can not exhibit higher oxidation states O form hydrogen bonding , Cl does not O is more electronegative and small in size than Cl Halogens are coloured Due to absorption of radiation from VR Iodine forms I3- but fluorine does not form F3Due to small size of fluorine HI can not be prepared by heating KI with conc. H2SO4 The magnitude of electron gain enthalpy of F is less than that of Cl Due to very small size of F there is inter electronic repulsion. Fluorine is stronger oxidizing agent than chlorine though it ha lower electron gain enthalpy Fluorine has higher reduction potential value due to its low bond dissociation energy and high hydration energy with compare to chlorine. Acid strength increases in the order HF< HCl < HBr < HI As size increases from F to Cl the bond dissociation energy decreases from HF to HI 29 GROUP-18 1 He , Ne do not form compound with F Due to high IE 2 Noble gases have very low b.pt Because there is only weak dispersion force between their atoms. 3 Hydrolysis of XeF6 is not a redox reaction Because in the products formed XeOF4 and XeO2F2 the Xe has the same oxidation state (+6) as in XeF6 4 Ne used as warning signal Because Ne – light has high fog penetration power 5 Noble gases form compounds only with fluorine and oxygen Because F & O are the most electronegative elements 6 Xe does not form XeF3 or XeF5 Xe has all paired electrons so promotion of one, two or three electrons will give rise to two, four or six unpaired electrons hence can not form XeF3 and XeF5 7 Out of noble gases only Xe forms compounds Because Xe has comparatively low IE and vacant orbitals for promotion of electrons 8 Noble gases are mostly inert Because they have completely filled valence orbitals i.e octet configuration 9 He is used as diving apparatus Because it is less soluble in blood with compare to nitrogen 10 It is difficult to study the chemistry of Rn Because Rn is radioactive and hence very unstable 11 Noble gases have comparatively large atomic size They are mono atomic so their van der Walls radii measured which is longer than covalent/ionic or metallic radii. 5 REACTION OF P BLOCK ELEMENTS 1. 2. 3. 4. 5. 3HNO2 → HNO3 + H2O + 2NO 41. Al2O3 + 6HCl + 9H2O → 2 [ Al(H2O)6 ]3+ + 6Cl− NH4CI(aq) + NaNO2(aq) → N2(g) +2H2O(l) + NaCl (aq) 42. 8NH3 + 3Cl2 → 6NH4Cl + N2; NH3 + 3Cl2 → NCl3 + 3HCl 43. PbS(s) + 4O3(g) → PbSO4(s) + 4O2(g) Ba(N3)2 → Ba + 3N2 44. 2I–(aq) + H O(l) + O (g) → 2OH–(aq) + I (s) + O (g) 2 6. 7. 8. 3 2 2 45. NO ( g ) + O ( g ) → NO ( g ) + O ( g ) 3 2 2 46. 4FeS (s ) + 11O ( g ) → 2Fe O3 ( s ) + 8SO ( g ) 2 2 2 2 47. SO2(g)+Cl2 (g) →SO2Cl2,SO2 + Ca(OH)2 + H2O +CaSO3 milky 48. 2Fe3+ + SO + 2H O → 2Fe2+ + SO2 − + 4H+ 2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2 (NH4)2 SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4 2 2 4 49. 5SO2+ 2MnO4 + 2H2O → 5SO42− + 4H+ + 2Mn2+ 9. ZnSO4( aq )+2NH4OH ( aq ) → Zn(OH)2 ( s ) + ( NH4)2 SO4 (aq )50. SO + H SO → H S O 3 2 4 2 2 7 10. FeCl3+NH4OH ( aq ) → Fe2O3 .xH2O (s) +NH4Cl ( aq ) 51. 11. Cu2+(aq) + 4NH3(aq) ‡ [Cu(NH3)4]2+ (aq) (blue) (deep blue) 52. 12. AgCl ( s ) + 2NH ( aq ) → Ag ( NH ) Cl ( aq ) 3 3 2 53. Cu + 2 H SO (conc.) → CuSO + SO + 2H O (white ppt) 2 (colourless) 4 4 2 2 13. 54. 3S + 2H SO (conc.) → 3SO + 2H O 2 4 2 2 55. C + 2H SO (conc.) → CO + 2 SO + 2 H O 14. 3NO ( g ) + H O ( l ) → 2HNO ( aq ) + NO ( g ) 2 2 3 15. 3Cu + 8 HNO (dilute) → 3Cu(NO ) + 2NO + 4H O 56. CaF + H SO → CaSO + 2HF 2 2 4 4 57. F + 2X– → 2F– + X (X = Cl, Br or I) 2 3 3 2 2 16. Cu + 4HNO (conc.) → Cu(NO ) + 2NO + 2H O 3 3 2 2 2 17. 4Zn + 10HNO (dilute) → 4 Zn (NO ) + 5H O + N O 3 3 2 2 18. Zn + 4HNO (conc.) → Zn (NO ) + 2H O + 2NO 3 3 2 2 2 19. I + 10HNO → 2HIO + 10NO + 4H O 2 3 3 2 2 20. C + 4HNO → CO + 2H O + 4NO 3 2 2 2 21. S + 48HNO → 8H SO + 48NO + 16H O 8 3 2 4 2 2 22. P + 20HNO → 4H PO + 20NO + 4H O 4 3 3 4 2 2 23. BROWN RING TEST 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 2 4 2 2 2 2 2 58. Cl + 2X– → 2Cl– + X (X = Br or I) 2 2 59. Br + 2I– → 2Br– + I 2 2 60. 2F (g) + 2H O (l) → 4H+ (aq) + 4F− (aq) + O (g) 2 2 2 ( where X = Cl or Br ) 61. 4I− (aq) + 4H+ (aq) + O (g) → 2I (s) + 2H O (l) 2 2 2 62. MnO + 4HCl → MnCl + Cl + 2H O 2 2 2 2 63. 4NaCl + MnO + 4H SO → MnCl +4NaHSO +2H O+Cl 2 2 4 2 4 2 2 64. 2KMnO + 16HCl → 2KCl + 2MnCl + 8H O + 5Cl 4 2 2 2 65. Cl + H O → 2HCl + O, Fe + 2HCl → FeCl + H 2 2 2 2 66. H S + Cl → 2HCl + S, NO3- + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O [Fe (H2O)6 ]2+ +NO→ [Fe(H2O)5 (NO)]2++ H2O 67. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2 68. P4 + 5O2 → P4O10 69. Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3 70. Ca3P2 + 6HCl → 3CaCl2 + 2PH3 71. PH4I + KOH → KI + H2O + PH3 72. 3CuSO4 + 2PH3 → Cu3 P2 + 3H2SO4 73. 3HgCl2 + 2PH3 → Hg3P2 + 6HCl 74. P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2 75. 3CH3COOH + PCl3 → 3CH3COCl + H3PO3 76. P4 + 10SO2Cl2 → 4PCl5 + 10SO2 77. PCl5+H2O → POCl3+2HCl, POCl3+3H2O →H3PO4+ 3HCl 78. S8 + 4Cl2 → 4S2Cl2 79. 2Ag + PCl5 → 2AgCl + PCl3 80. PCl3 + 3H2O →H3PO3 + 3HCl 81. 4H3PO3 → 3H3PO4 + PH3 2 2 Au + 4H+ + NO3− + 4Cl− → AuCl−4 + NO + 2H2O 3Pt + 16H+ + 4NO3 + 18Cl− → 3PtCl6− + 4NO + 8H2O (cold and dilute)2NaOH + Cl2 → NaCl + NaOCl + H2O (hot and conc.)6 NaOH + 3Cl2 →5NaCl +NaClO3 + 3H2O 2Ca(OH)2 + 2Cl2 → Ca(OCl)2 + CaCl2 + 2H2O I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl 2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl SO2 + 2H2O + Cl2 → H2SO4 + 2HCl XeF4 + O2 F2 → XeF6 + O2 2XeF2 (s) + 2H2O(l) → 2Xe (g) + 4 HF(aq) + O2(g) 6XeF4 + 12 H2O → 4Xe + 2XeO3 + 24 HF + 3 O2 XeF6+H2O→XeOF4 + 2 HF,XeF6+2H2O →XeO2F2 + 4HF XeF6 + 3 H2O → XeO3 + 6 HF 2NH3 + 3NaClO →N2 + 3H2O + 3NaCl 82. 2K2HgI4 + NH3 + 3KOH→ Brown ppt. of HN HgOHgI 2 83. 2NH3 + NaOCl →NH2.NH2 hydrazine Al2O3 ( s )+6NaOH(aq)+3H2O ( l ) → 2Na3[Al(OH)6](aq) REACTION OF P BLOCK ELEMENTS 1. 2. 3. 4. 5. 3HNO2 → HNO3 + H2O + 2NO 41. Al2O3 + 6HCl + 9H2O → 2 [ Al(H2O)6 ]3+ + 6Cl− NH4CI(aq) + NaNO2(aq) → N2(g) +2H2O(l) + NaCl (aq) 42. 8NH3 + 3Cl2 → 6NH4Cl + N2; NH3 + 3Cl2 → NCl3 + 3HCl 43. PbS(s) + 4O3(g) → PbSO4(s) + 4O2(g) Ba(N3)2 → Ba + 3N2 44. 2I–(aq) + H O(l) + O (g) → 2OH–(aq) + I (s) + O (g) 2 6. 7. 8. 3 2 2 45. NO ( g ) + O ( g ) → NO ( g ) + O ( g ) 3 2 2 46. 4FeS (s ) + 11O ( g ) → 2Fe O3 ( s ) + 8SO ( g ) 2 2 2 2 47. SO2(g)+Cl2 (g) →SO2Cl2,SO2 + Ca(OH)2 + H2O +CaSO3 milky 48. 2Fe3+ + SO + 2H O → 2Fe2+ + SO2 − + 4H+ 2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2 (NH4)2 SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4 2 2 4 49. 5SO2+ 2MnO4 + 2H2O → 5SO42− + 4H+ + 2Mn2+ 9. ZnSO4( aq )+2NH4OH ( aq ) → Zn(OH)2 ( s ) + ( NH4)2 SO4 (aq )50. SO + H SO → H S O 3 2 4 2 2 7 10. FeCl3+NH4OH ( aq ) → Fe2O3 .xH2O (s) +NH4Cl ( aq ) 51. 11. Cu2+(aq) + 4NH3(aq) ‡ [Cu(NH3)4]2+ (aq) (blue) (deep blue) 52. 12. AgCl ( s ) + 2NH ( aq ) → Ag ( NH ) Cl ( aq ) 3 3 2 53. Cu + 2 H SO (conc.) → CuSO + SO + 2H O (white ppt) 2 (colourless) 4 4 2 2 13. 54. 3S + 2H SO (conc.) → 3SO + 2H O 2 4 2 2 55. C + 2H SO (conc.) → CO + 2 SO + 2 H O 14. 3NO ( g ) + H O ( l ) → 2HNO ( aq ) + NO ( g ) 2 2 3 15. 3Cu + 8 HNO (dilute) → 3Cu(NO ) + 2NO + 4H O 56. CaF + H SO → CaSO + 2HF 2 2 4 4 57. F + 2X– → 2F– + X (X = Cl, Br or I) 2 3 3 2 2 16. Cu + 4HNO (conc.) → Cu(NO ) + 2NO + 2H O 3 3 2 2 2 17. 4Zn + 10HNO (dilute) → 4 Zn (NO ) + 5H O + N O 3 3 2 2 18. Zn + 4HNO (conc.) → Zn (NO ) + 2H O + 2NO 3 3 2 2 2 19. I + 10HNO → 2HIO + 10NO + 4H O 2 3 3 2 2 20. C + 4HNO → CO + 2H O + 4NO 3 2 2 2 21. S + 48HNO → 8H SO + 48NO + 16H O 8 3 2 4 2 2 22. P + 20HNO → 4H PO + 20NO + 4H O 4 3 3 4 2 2 23. BROWN RING TEST 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 2 4 2 2 2 2 2 58. Cl + 2X– → 2Cl– + X (X = Br or I) 2 2 59. Br + 2I– → 2Br– + I 2 2 60. 2F (g) + 2H O (l) → 4H+ (aq) + 4F− (aq) + O (g) 2 2 2 ( where X = Cl or Br ) 61. 4I− (aq) + 4H+ (aq) + O (g) → 2I (s) + 2H O (l) 2 2 2 62. MnO + 4HCl → MnCl + Cl + 2H O 2 2 2 2 63. 4NaCl + MnO + 4H SO → MnCl +4NaHSO +2H O+Cl 2 2 4 2 4 2 2 64. 2KMnO + 16HCl → 2KCl + 2MnCl + 8H O + 5Cl 4 2 2 2 65. Cl + H O → 2HCl + O, Fe + 2HCl → FeCl + H 2 2 2 2 66. H S + Cl → 2HCl + S, NO3- + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O [Fe (H2O)6 ]2+ +NO→ [Fe(H2O)5 (NO)]2++ H2O 67. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2 68. P4 + 5O2 → P4O10 69. Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3 70. Ca3P2 + 6HCl → 3CaCl2 + 2PH3 71. PH4I + KOH → KI + H2O + PH3 72. 3CuSO4 + 2PH3 → Cu3 P2 + 3H2SO4 73. 3HgCl2 + 2PH3 → Hg3P2 + 6HCl 74. P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2 75. 3CH3COOH + PCl3 → 3CH3COCl + H3PO3 76. P4 + 10SO2Cl2 → 4PCl5 + 10SO2 77. PCl5+H2O → POCl3+2HCl, POCl3+3H2O →H3PO4+ 3HCl 78. S8 + 4Cl2 → 4S2Cl2 79. 2Ag + PCl5 → 2AgCl + PCl3 80. PCl3 + 3H2O →H3PO3 + 3HCl 81. 4H3PO3 → 3H3PO4 + PH3 2 2 Au + 4H+ + NO3− + 4Cl− → AuCl−4 + NO + 2H2O 3Pt + 16H+ + 4NO3 + 18Cl− → 3PtCl6− + 4NO + 8H2O (cold and dilute)2NaOH + Cl2 → NaCl + NaOCl + H2O (hot and conc.)6 NaOH + 3Cl2 →5NaCl +NaClO3 + 3H2O 2Ca(OH)2 + 2Cl2 → Ca(OCl)2 + CaCl2 + 2H2O I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl 2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl SO2 + 2H2O + Cl2 → H2SO4 + 2HCl XeF4 + O2 F2 → XeF6 + O2 2XeF2 (s) + 2H2O(l) → 2Xe (g) + 4 HF(aq) + O2(g) 6XeF4 + 12 H2O → 4Xe + 2XeO3 + 24 HF + 3 O2 XeF6+H2O→XeOF4 + 2 HF,XeF6+2H2O →XeO2F2 + 4HF XeF6 + 3 H2O → XeO3 + 6 HF 2NH3 + 3NaClO →N2 + 3H2O + 3NaCl 82. 2K2HgI4 + NH3 + 3KOH→ Brown ppt. of HN HgOHgI 2 83. 2NH3 + NaOCl →NH2.NH2 hydrazine Al2O3 ( s )+6NaOH(aq)+3H2O ( l ) → 2Na3[Al(OH)6](aq) 7 REACTION OF P BLOCK ELEMENTS 1. 2. 3. 4. 5. 3HNO2 → HNO3 + H2O + 2NO 41. Al2O3 + 6HCl + 9H2O → 2 [ Al(H2O)6 ]3+ + 6Cl− NH4CI(aq) + NaNO2(aq) → N2(g) +2H2O(l) + NaCl (aq) 42. 8NH3 + 3Cl2 → 6NH4Cl + N2; NH3 + 3Cl2 → NCl3 + 3HCl 43. PbS(s) + 4O3(g) → PbSO4(s) + 4O2(g) Ba(N3)2 → Ba + 3N2 44. 2I–(aq) + H O(l) + O (g) → 2OH–(aq) + I (s) + O (g) 2 6. 7. 8. 3 2 2 45. NO ( g ) + O ( g ) → NO ( g ) + O ( g ) 3 2 2 46. 4FeS (s ) + 11O ( g ) → 2Fe O3 ( s ) + 8SO ( g ) 2 2 2 2 47. SO2(g)+Cl2 (g) →SO2Cl2,SO2 + Ca(OH)2 + H2O +CaSO3 milky 48. 2Fe3+ + SO + 2H O → 2Fe2+ + SO2 − + 4H+ 2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2 (NH4)2 SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4 2 2 4 49. 5SO2+ 2MnO4 + 2H2O → 5SO42− + 4H+ + 2Mn2+ 9. ZnSO4( aq )+2NH4OH ( aq ) → Zn(OH)2 ( s ) + ( NH4)2 SO4 (aq )50. SO + H SO → H S O 3 2 4 2 2 7 10. FeCl3+NH4OH ( aq ) → Fe2O3 .xH2O (s) +NH4Cl ( aq ) 51. 11. Cu2+(aq) + 4NH3(aq) ‡ [Cu(NH3)4]2+ (aq) (blue) (deep blue) 52. 12. AgCl ( s ) + 2NH ( aq ) → Ag ( NH ) Cl ( aq ) 3 3 2 53. Cu + 2 H SO (conc.) → CuSO + SO + 2H O (white ppt) 2 (colourless) 4 4 2 2 13. 54. 3S + 2H SO (conc.) → 3SO + 2H O 2 4 2 2 55. C + 2H SO (conc.) → CO + 2 SO + 2 H O 14. 3NO ( g ) + H O ( l ) → 2HNO ( aq ) + NO ( g ) 2 2 3 15. 3Cu + 8 HNO (dilute) → 3Cu(NO ) + 2NO + 4H O 56. CaF + H SO → CaSO + 2HF 2 2 4 4 57. F + 2X– → 2F– + X (X = Cl, Br or I) 2 3 3 2 2 16. Cu + 4HNO (conc.) → Cu(NO ) + 2NO + 2H O 3 3 2 2 2 17. 4Zn + 10HNO (dilute) → 4 Zn (NO ) + 5H O + N O 3 3 2 2 18. Zn + 4HNO (conc.) → Zn (NO ) + 2H O + 2NO 3 3 2 2 2 19. I + 10HNO → 2HIO + 10NO + 4H O 2 3 3 2 2 20. C + 4HNO → CO + 2H O + 4NO 3 2 2 2 21. S + 48HNO → 8H SO + 48NO + 16H O 8 3 2 4 2 2 22. P + 20HNO → 4H PO + 20NO + 4H O 4 3 3 4 2 2 23. BROWN RING TEST 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 2 4 2 2 2 2 2 58. Cl + 2X– → 2Cl– + X (X = Br or I) 2 2 59. Br + 2I– → 2Br– + I 2 2 60. 2F (g) + 2H O (l) → 4H+ (aq) + 4F− (aq) + O (g) 2 2 2 ( where X = Cl or Br ) 61. 4I− (aq) + 4H+ (aq) + O (g) → 2I (s) + 2H O (l) 2 2 2 62. MnO + 4HCl → MnCl + Cl + 2H O 2 2 2 2 63. 4NaCl + MnO + 4H SO → MnCl +4NaHSO +2H O+Cl 2 2 4 2 4 2 2 64. 2KMnO + 16HCl → 2KCl + 2MnCl + 8H O + 5Cl 4 2 2 2 65. Cl + H O → 2HCl + O, Fe + 2HCl → FeCl + H 2 2 2 2 66. H S + Cl → 2HCl + S, NO3- + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O [Fe (H2O)6 ]2+ +NO→ [Fe(H2O)5 (NO)]2++ H2O 67. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2 68. P4 + 5O2 → P4O10 69. Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3 70. Ca3P2 + 6HCl → 3CaCl2 + 2PH3 71. PH4I + KOH → KI + H2O + PH3 72. 3CuSO4 + 2PH3 → Cu3 P2 + 3H2SO4 73. 3HgCl2 + 2PH3 → Hg3P2 + 6HCl 74. P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2 75. 3CH3COOH + PCl3 → 3CH3COCl + H3PO3 76. P4 + 10SO2Cl2 → 4PCl5 + 10SO2 77. PCl5+H2O → POCl3+2HCl, POCl3+3H2O →H3PO4+ 3HCl 78. S8 + 4Cl2 → 4S2Cl2 79. 2Ag + PCl5 → 2AgCl + PCl3 80. PCl3 + 3H2O →H3PO3 + 3HCl 81. 4H3PO3 → 3H3PO4 + PH3 2 2 Au + 4H+ + NO3− + 4Cl− → AuCl−4 + NO + 2H2O 3Pt + 16H+ + 4NO3 + 18Cl− → 3PtCl6− + 4NO + 8H2O (cold and dilute)2NaOH + Cl2 → NaCl + NaOCl + H2O (hot and conc.)6 NaOH + 3Cl2 →5NaCl +NaClO3 + 3H2O 2Ca(OH)2 + 2Cl2 → Ca(OCl)2 + CaCl2 + 2H2O I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl 2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl SO2 + 2H2O + Cl2 → H2SO4 + 2HCl XeF4 + O2 F2 → XeF6 + O2 2XeF2 (s) + 2H2O(l) → 2Xe (g) + 4 HF(aq) + O2(g) 6XeF4 + 12 H2O → 4Xe + 2XeO3 + 24 HF + 3 O2 XeF6+H2O→XeOF4 + 2 HF,XeF6+2H2O →XeO2F2 + 4HF XeF6 + 3 H2O → XeO3 + 6 HF 2NH3 + 3NaClO →N2 + 3H2O + 3NaCl 82. 2K2HgI4 + NH3 + 3KOH→ Brown ppt. of HN HgOHgI 2 83. 2NH3 + NaOCl →NH2.NH2 hydrazine Al2O3 ( s )+6NaOH(aq)+3H2O ( l ) → 2Na3[Al(OH)6](aq) 8