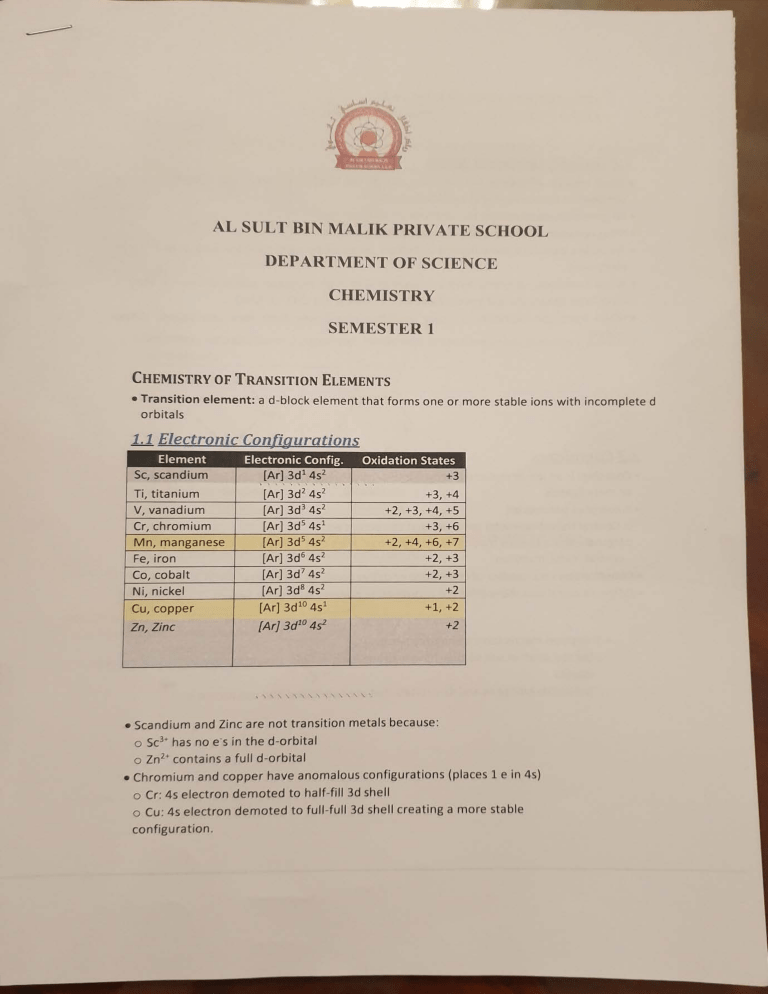
... AL SULT BIN MALIK PRIVATE SCHOOL DEPARTMENT OF SCIENCE CHEMISTRY SEMESTER 1 CHEMISTRY OF TRANSITION ELEMENTS • Transition element: a d-block element that forms one or more stable ions with incomplete d orbita Is 1.1 Electronic Configurations Element Sc, scandium Electronic Config. [Ar] 3d 1 4s2 , , .. , .. , .. .. , 2 2 .. Oxidation States ' +3 ' . Ti, titanium V, vanadium [Ar] 3d 4s [Ar] 3d 3 4s2 Cr, chromium Fe, iron [Ar] 3d 5 4s1 [Ar] 3d 5 4s 2 [Ar] 3d 6 4s 2 Co, cobalt [Ar] 3d 7 4s2 +3,+4 +2,+3,+4,+S +3,+6 +2,+4,+6, +7 +2,+3 +2,+3 Ni, nickel (Ar] 3d 8 4s 2 +2 Cu,coppe r [Ar] 3d 10 4s 1 +1, +2 Zn, Zinc [Ar] 3d 10 4s2 +2 Mn, manganese • Scandium and Zinc are not transition metals because: o Sc 3• has no e-s in the d -orbital o Zn 2• contains a full d-orbital • Chromium and copper have anomalous configurations (places 1 e in 4s) o Cr: 4s electron demoted to half-fill 3d shell 0 Cu: 4s electron demoted to full -full 3d shell creating a more stable configuration . • When electron dd d, fill b to, , • When I ctron r mov d, r mov . r on, Lis b ror 3d 1.2 Variab,le O idatio11 Sta e • Small energy diff r -nee betwe · n 4s and 3d so el c rans from both subshells can be removed to form a variety of oxidation sta tes 1 1 • All transition metar exhibit two or more oxidatio,n states • Most common o idation state +2 when 2e s from 4s lost • Transition elements show h,ighest oxidation states whe,n they combine with O or F (most electro-ve) • When tra ,n sition elemen1ts form compounds with high oxidation states above +4, they form large oxoanions and are covalent (aci'dic o,xides) e.g. CrO - or Mn,Q · 1 • When transition1 elements in lowe,r oxidation stat,es they form io,nic compounds (basiCA oxides) 1.3 Complexes • Complex: is a,n .ion or molecule formed by a central m,etal atom/ ion surrounded by o,ne or more ligands • A complex consists of.: o Central transition metal ion ,(+ve) that c.an accept e·s a Ligand (-ve): a specie,s that c,ontains a lone pair of e~s that forms a dative bond to a central metal atom/,ion • Coordination no.: number of coordinate or dative bo,nds t ,o the central meta i atom/ion • Different metal ions show different ,coordination number with same liga .nds • Transition metals form complexes because Ion are small rn size so they hav,e a strong electric field ,around them which attract e·-rich 0 ligands They have empty 4s and 4p orbitals that are hybridised and can accept ,e· 0 4 1.4 Ligand~ • Monodentate ligands· forms o nl pair of es) . Anions Halide ions F, c1·, Br·, r Sulphide 52N02, . Nitrite Hydroxide OH· Cyanide CN· Thiocyanate SCN· . y one coo rdin ate bon d wi th centra l meta l ion (donates one Neutral Ligands Water H20 Ammonia NH3 Carbonyl co • Bidentate ligands: forms 2 coordinate bonds with central metal ion (dona t es 2 pairs of e·s per molecule) H2 C CH 2 \ I H2 N ~ / M 0 0 11 11 C C \ I NH 2 0 ~ / M 0 H2N - CH2 - CH2 - NH2 ·o - co - co - o· Ethylene diamine (en) (neutral) Oxalate ion (oxalate-) • Polydentate ligands: forms 2 or more coordinate bonds with central metal ion -•O OCCH2 . ) -:ooccH2 / -. Ethylene Dia mine Tetra Acetic Acid (EDTA) / C H2COO. 0 \\ NCH2CH2N: "" CH2coo:- o Forms 6 coordinate bonds - 4 from oxygen - 2 from nitrogen 0 •M • Pl 0 Ii 11 f I tl r II t c t I If Ii r1d lJ I rn u • I Jll1 r I I r I k lJ n II r1 d L , 11 tl on t1 I m I - 11 Ill h rg on ligand l I ions n 011 m t I ion Diagram Coordination No. & Shape H H H .O • .o. •• , , ..,.a / 6 Octahedral •• H / O: H . H [Fe,(Hzi0 )6] 2 2- Cl •• 4 Tetrahedra l ,, Co C ~-- - • •c1 Cl•• [CoC'l4) 2- 2- .c ---·- ·• ., c.. 4 s.qua re Planar (most Ni and Pt) Jl '# c·/_ • - - -- - •( H 2 Linear H I LZ S tereoiso111e1·is111 Geometric isomerism (els-trans) Cl . NH3 ·.. Pt . Cl......... H3N. . Cl ·.. Pt .. " ' N H3 Cl_....- cis-platin "NH3 trans-platin • C!s~~latin is an anticancer drug that acts by binding to DNA in cancer cells, preventing cell d1v1s1on 1.1 Common Complexes Ligand H20 NH3 (drops) NH3(excess) OH· Cu(//) [Cu(H20)6] 2+ 1 1 I Cu(H20)4(0H)2 [ Cu(N H3)4( H20 )2] 2+ Cu(H20)4(QH)2 Cl· [CuCl4J 2• Co(II) [Co(H20)6] 2+ Co(H20)4(QH)2 [Co(NH3)6] 2+ Co(H20)4(QH)2 [CoCl4J 2· Copper Chemistry 1. Reaction with Hydroxide Ions (OH-ions from alkali eg.NaOH) Cu (l120) 4 (OH) 2 + )f · Not ligand exchange; hydroxide ions remove hydrogen ions from water ligand, this is a deprotonation reaction . 2. Reaction with Ammonia Solution Small amount of ammonia 11 (11 )) (lfl) I) · In it i II , r n1 , i E I 1n t b 1 II [ 'u l\l IJ t<I m · Only 3 r I ,uII I ff t f1 I X u on bov . I I u H . n i r1 t d t I If 1 ) '] ~1 l1lu( a l'igand at r n1 I u le r plac d f the ction ith hloride Ions (Cl-ions from HCI) 4 1- + I I · 6 water morecures replaced by 4 chloride ions ote: A green so lution is observed, however, the colour of [CuCl1] 2blue Cu (H20) 6] 2- and the yellow [CuCl4 ] 2- is yellow, this is because, both the are present in the equilibrium mixture. Cobalt Chemistry Reaction with Hydroxide Ions (OH-ions from alkali eg.NaOH) 1. OH- Co (H20) 4 (OH) 2 + · Not ligand exchange; hydroxide ions remove hydrogen ions from water ligand, this is a deprotonation reaction. 2. Reaction with Ammonia Solution Small amount of ammonia 2 H3 Co (l I20) 4 (OI I) 2 2[ H1 · Initially, ammonin act s as a llase and hyrfrc> • gen ions drro PIIIIPcl c>f f lh(• "" «•• ,1 h() /(> Xt1,1q11,1 l<lrl .. , . Excess ommon,a rco (JI O) (c·o(Nlla),, 12 · Ammonia repla ces water as a ligand th e com le . Co (~l13) i h1 h , P x ion formed oxidises in air to losr an electron and • u w c 1s dark solution. form a complex 3. Reaction with Chloride Ions (Cl- ions from HCI) [Co (fi20) 6 2 + ....... + 611.d) . 6 water molecules replaced by 4 chloride ions l igand Exchange and Stability Constant · Ligand exchange; a more powerful ligand will substitute a less powerful ligand from a cation of the complex and this can produce a change in colour and shape Strength of Different Ligands; EDTA>S >CN >I >52032 >Br>NH3> CI >H20 Strongest weakest · Exchange of ligands can be explained in terms of completing equilibria of forward & backward reaction · Equilibrium position lies towards more stable complex · Adding excess weak ligand can shift equilibrium backward and form weaker complex




