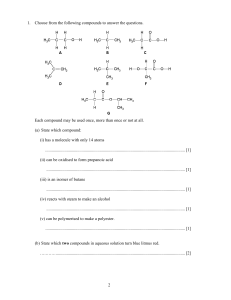
Year 11 Chemical Changes - Homework 2 Score:___/19 Name:________ 1) Define the following terms: Oxidation: _____________________________________________________________ Reduction: _____________________________________________________________ Anion:_________________________________________________________________ Cation:_________________________________________________________________ Anode: ________________________________________________________________ (4) 2) Explain why solid MgCl2 cannot be electrolysed. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ (2) 3) Complete the following half equations: i) Na+ + ___ → Na iii) Mg2+ + ___ → ___ ii) ___F- → F2 + ___ iv) ___Cl- → ___ + ___ (4) 4) Explain why potassium cannot be extracted from its ore by smelting? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ (2) 5) Write a word equation for the reaction of copper oxide and carbon to form copper metal and carbon dioxide. ______________________________________________________________________ (1) 6) Write a symbol equation for the reaction of hydrochloric acid with sodium hydroxide to form sodium chloride and one other product ______________________________________________________________________ (2) 7) Describe the product formed at each electrode when an aqueous solution of KCl is electrolysed. Give a half equation for the reaction at each electrode. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ (4)