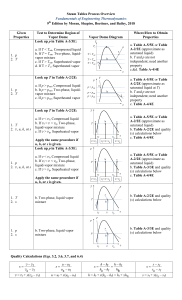
PROPERTIES OF A PURE SUBSTANCE MEC121 LECTURE 2 Pure substance: a material with homogeneous and invariable composition. • Pure substances can have multiple phases: an ice-water mixture is still a pure substance. • An air-steam mixture is not a pure substance. • Air, being composed of a mixture of N2, O2, and other gases, is formally not a pure substance. But can often treat air as a pure substance with little error. Temperature − Specific Volume Temperature − Specific Volume Above a critical pressure, P = Pc = 22.089 MPa, there is no phase change observed!1 At the critical pressure, the temperature takes on a critical temperature of Tc = 374.14 ◦C. At the critical pressure and temperature, the specific volume takes the value vf = vg = vc = 0.003155 m3/kg. saturated liquid: the material is at Tsat and is all liquid. saturated vapor: the material is at Tsat and is all vapor. compressed (subcooled) liquid: the material is liquid with T < Tsat. superheated vapor: the material is vapor with T > Tsat. two-phase mixture: the material is composed of co-existing liquid and vapor with both at Tsat Quality (x) • quality= x: as the ratio of the mass of the mixture that is vapor (vap) to the total mixture mass: x = mvap/mtotal . x = 0: corresponds to mvap = 0. This is the all liquid limit. x = 1: corresponds to mvap = mtotal. This is the all gas limit. x ∈ [0, 1]. Independent properties • Simple compressible substance: a material that can be worked upon by pressure forces. For a simple compressible substance, two independent intensive thermodynamic properties define the state of the system. superheated vapor /compressed(subcooled) liquid: P = P(T, v), v = v(T, P), or T = T(P, v). two-phase mixture: If we have a two-phase mixture, our experiments show that P and T are not independent. In this case, we need another property to characterize the system. That property is the quality, x. So for two-phase mixtures, we might have v = v(T, x), for example. • Thermal equation of state: an equation that gives the pressure as a function of two independent state variables. An example is the general form: Saturated Table (water) Compressed Liquid Table Superheated Table Linear interpolation • Interpolation is often required when exact values are not tabulated. • primarily use linear interpolations. • Use extrapolations only if there is no other choice. • Occasionally double interpolations will be necessary. Interpolation Thermodynamic Surfaces Ideal gas • Ideal gas law: This equation, which is a combination of Boyle’s law, Charles’ law, and Avogadro’s law, is most fundamentally stated as P V = nṜT Ideal Gas • Ř is independent of material (universal gas constant). • M; molecular weight of gas, • R ; gas constant of a particular gas. Compressibility factor Ideal Gas Example: Given air in a cylinder with stops and a frictionless piston with area A = 0.2 m2 , stop height of 1 m, and total height of 2 m, at initial state P1 = 200 kPa and T1 = 500 ◦C with cooling, find 1. the temperature when the piston reaches the stops, and •2. the pressure if the cooling continues to T = 20 ◦C. three distinct states: • state 1: initial state •state 2: piston reaches the stops • state 3: final state, where T = 20◦C. Ideal Gas Ideal Gas