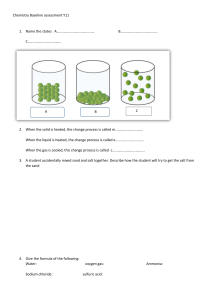
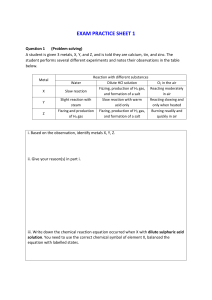
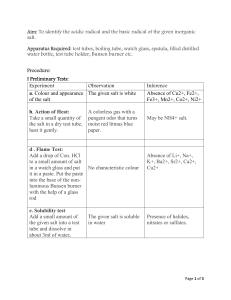
More concentration/dilution worksheet Concentration 1. Calculate the following concentrations in g/L a) 15 g of NaNO3 in 750 mL of solution b) 25 g of LiOH in 3.5 L of solution c) 50 % m/V of AgNO3 d) 6 g of HF in 250 mL of solution 2. Marco has three solutions of potassium chloride. Which solution has the highest concentration? Solution 1: 8 g in 0.35 L Solution 2: 2 g in 540 mL Solution 3: 279.75 g in 14 L 3. The following three solutions show different concentrations of calcium chloride (CaCl2) in water. Which solution has the highest concentration? Solution 1: 0.2 kg in 0.8 L Solution 2: 185 g in300 mL Solution 3: 75 g in 0.25 L 4. Katia has a variety of different drinks. Calculate the sugar concentration of each drink and rank them from lowest to highest. Bubbly: 25 g in 1.2 L Fizzy pop: 10 % m/V Hummingbird soda: 10 g in 250 mL Red eye cola: 1.5 g in 0.1 L 5. Convert 5.3 % m/V into g/ml and g/L. Changes in Concentration - Dilution 1. I have a 0.53 mL solution of salt water with a concentration of 12 g/L. I add 8 g of salt. What is the new concentration? 2. I have an 0.6 L solution of sugar water with a concentration of 0.36 g/mL. I add 240 mL of water. What is the new concentration? 3. Solution A: 30mL of salt water with concentration 4.70 g/L. Solution B: 20mL of salt water with concentration 42.8g/L. If I mix the two solutions, what will be the new concentration? 4. Solution A: 5400 mL of salt water with concentration 87 g/L. Solution B: 1.84 L of distilled water. If I mix the two solutions, what will be the new concentration? 5. I have a 900 mL solution of sulfuric acid with a concentration of 140 g/L. I want to dilute it to get a solution with a concentration of 35 g/L. How much water do I need to add? 6. I have a 0.75 L solution of sulfuric acid with a concentration of 14 g/L. I want to dilute it to get a solution with a concentration of 5.6 g/L. How much water do I need to add?