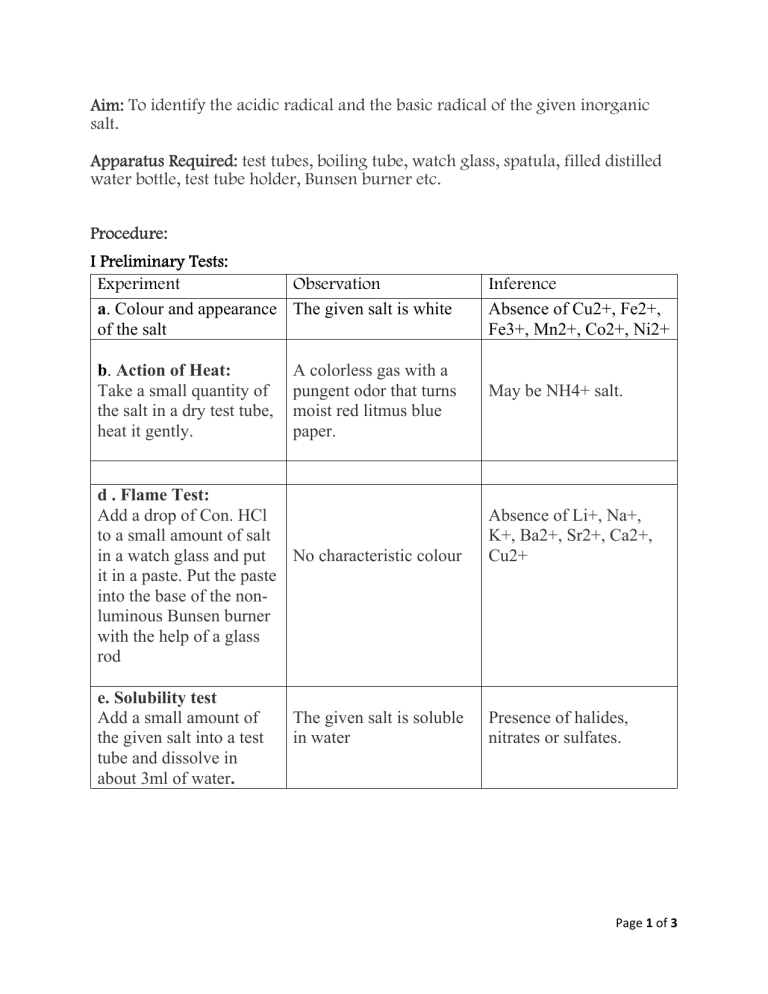
Aim: To identify the acidic radical and the basic radical of the given inorganic
salt.
Apparatus Required: test tubes, boiling tube, watch glass, spatula, filled distilled
water bottle, test tube holder, Bunsen burner etc.
Procedure:
I Preliminary Tests:
Experiment
Observation
a. Colour and appearance The given salt is white
of the salt
b. Action of Heat:
Take a small quantity of
the salt in a dry test tube,
heat it gently.
A colorless gas with a
pungent odor that turns
moist red litmus blue
paper.
d . Flame Test:
Add a drop of Con. HCl
to a small amount of salt
in a watch glass and put
No characteristic colour
it in a paste. Put the paste
into the base of the nonluminous Bunsen burner
with the help of a glass
rod
e. Solubility test
Add a small amount of
the given salt into a test
tube and dissolve in
about 3ml of water.
The given salt is soluble
in water
Inference
Absence of Cu2+, Fe2+,
Fe3+, Mn2+, Co2+, Ni2+
May be NH4+ salt.
Absence of Li+, Na+,
K+, Ba2+, Sr2+, Ca2+,
Cu2+
Presence of halides,
nitrates or sulfates.
Page 1 of 3
II. Systematic analysis of anions (acid radicals)
Experiment
a. Action of
dilute.H2SO4:
Add 1 or 2ml of dilute
H2SO4 to a small portion
of the salt in a test tube
and warm it gently.
b. Action of Con.
H2SO4:
Add 2-3 ml of Con.
H2SO4 to a small amount
of salt taken in a test tube
and heat it gently.
c. Potassium
permanganate test
To a pinch of salt in test
tube add about 2ml of
dilute sulphuric acid.
Boil off any gas evolved,
add a little more dilute
acid and the KMnO4
solution dropwise.
Observation
Inference
No characteristic
observation.
Absence of CO2-, S2-,
SO42-, NO32-, CH3COO-
Colorless gas with a
pungent smell that
provides dense white
fumes with a glass rod
dipped
in NH4OH solution.
May be chloride anion.
KMnO4 decolourised in
cold with the evolution of May be chloride anion
a gas with bleach odour
Page 2 of 3
III. Confirmatory Test for Chloride ion
Experiment
a. Silver nitrate test
Acidify a portion of
aqueous solution of the
salt in a test tube with dil.
HNO3. Boil for some
time, cool and add silver
nitrate solution.
b. Manganese dioxide
(MnO2)
Heat a pinch of the salt
with small quantity of
MnO2 and con. H2SO4
Observation
Inference
A white precipitate is
formed which is soluble
in NH4OH {aq.
Ammonia)
Presence of Cl- ion
confirmed.
Evolution of greenish
yellow gas having a
pungent irritating smell
which turns moist starch
iodide paper blue.
Presence of Cl- ion
confirmed.
IV. Confirmatory Test for ammonium ion
Experiment
a. Action of NaOH:
Take a small quantity of
the salt in a dry test tube,
heat it gently with con.
NaOH solution.
Observation
A colorless gas with a
pungent evolved which
gives white fumes when
a glass rod dipped in dil.
HClis brought near the
mouth of the test tube.
b. Nessler’s reagent test
Add a few drops of the
A brown ppt formed.
salt solution to 2ml of
Nessler’s reagent taken in
a test tube.
Inference
Presence of ammonium
ion confirmed
Presence of NH4+ ion
confirmed.
Result:
Acid radical present in the given salt is chloride ion (Cl-).
Basic radical present in the given salt is Ammonium ion (NH4 +).
Page 3 of 3



