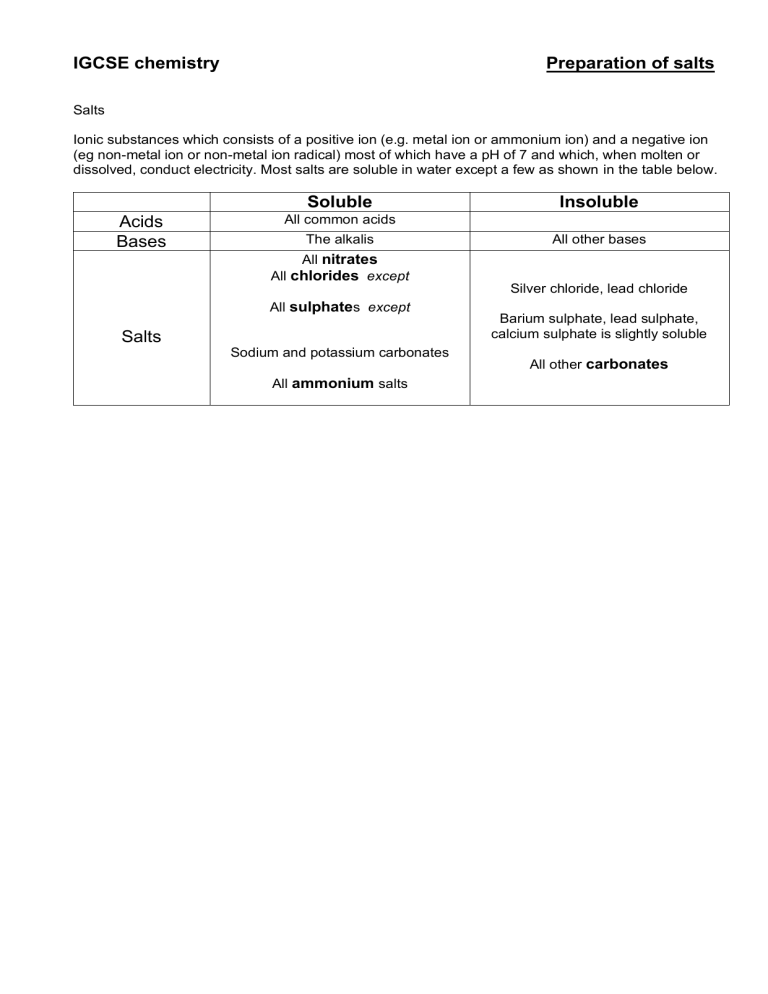
IGCSE chemistry Preparation of salts Salts Ionic substances which consists of a positive ion (e.g. metal ion or ammonium ion) and a negative ion (eg non-metal ion or non-metal ion radical) most of which have a pH of 7 and which, when molten or dissolved, conduct electricity. Most salts are soluble in water except a few as shown in the table below. Acids Bases Soluble Insoluble All common acids The alkalis All other bases All nitrates All chlorides except Silver chloride, lead chloride All sulphates except Salts Sodium and potassium carbonates All ammonium salts Barium sulphate, lead sulphate, calcium sulphate is slightly soluble All other carbonates How do we make samples of pure dry crystals of a salt. The flowchart below will help you to identify the correct method no Is salt soluble? yes Base soluble? no yes 1. For each of the following salts: decide which method you will use to make the salt and select suitable chemicals; Salt you are going to make Starting chemicals you will use The method you will use magnesium sulfate sodium nitrate calcium chloride barium chloride ammonium nitrate copper ethanoate 2. This question is about preparing insoluble salts. (a) Complete the table below about he making of insoluble salts 1. 2. 3. 4. 5. salt copper carbonate silver chloride lead sulphate lead chloride barium sulphate starting chemicals (b) For each of the above examples (i) write a chemical equation and (ii) an ionic equation. 3. Add these captions in the correct boxes on the flowchart. Precipitation Neutralization (Acid + excess base/metal) - Make solutions containing ions of salt to be made (need soluble salts) - Add the solutions - Filter off precipitate - Wash precipitate with distilled water - Dry with filter paper or on warm gauze - Add excess base/metal to acid until no more dissolves (warm acid if necessary) - filter off excess base/metal - obtain salt from solution (see below) Indicator method/titration Obtaining dry salt from solution - Add acid via burette to alkali` + indicator until neutral ( or until temperature stops rising) - Measure volume of acid added; throw away solution; - Add same volume of acid to same amount of alkali without indicator - Obtain dry salt from solution - Concentrate solution until it becomes saturated which is done by driving off most of the water by heating it. Solution is saturated when crystals form on a glass rod dipped in the solution. - Stop heating and allow solution to cool - Crystals can be washed with distilled water - Dry crystals with filter paper or place on warm gauze or in warm place 4. Complete the following symbol and word equations of neutralization reactions (you will need to balance the symbol equations): ⎯→ a. HCl (aq) + MgO (s) b. H2SO4 (aq) + KOH (aq) ⎯→ c. HNO3 (aq) + NH4OH (aq) ⎯→ d. C2H3OOH(aq) e. HNO3 (aq) NaOH (aq) ⎯→ + + Ca(OH)2 (aq) ⎯→ f. sulfuric acid (aq) + copper oxide (s) ⎯→ g. hydrochloric acid (aq) + lithium oxide (s) ⎯→ h. nitric acid (aq) + copper oxide (s) ⎯→ i. ⎯→ CaSO4 (aq) + H2O (l) j. ⎯→ ZnCl2 (aq) + H2O (l) 5. The diagram below shows some reactions of dilute sulfuric acid. Use it to answer the questions below. magnesium ribbon copper (II) oxide magnesium sulfate solution + gas A blue solution B sodium hydrogen carbonate sulfuric acid sodium sulfate solution + gas C substance D substance D goes red solution E potassium sulfate solution only Name or give the formula of each of the following : (a) (b) (c) (d) (e) gas A: solution B: gas C: substance D: solution E:






