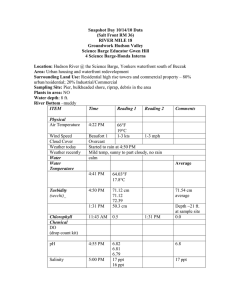
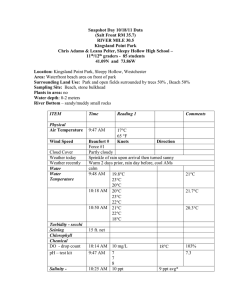
Problem 1 1 L of water is analyzed for the magnesium chloride. The analysis indicates that the sample contains 5 mg of the salt. What is the salt concentration in g/L, mg/L, ppm, ppb, ppt, M, mM, µM, nM and picoM? Solution 1. 5 mg/L = 5 ppm 5×10−3 g/L 5000 ppb 5 000 000 ppt = 5×106 ppt 2. MW (MgCl2) = 95 g/mol 𝐶𝑚 CM = 𝑀𝑊 = 5 ×10−3 95 =0.0000526 M 0.0526 mM 52.6 µM 52 600 nM 52 600 000 pM 1