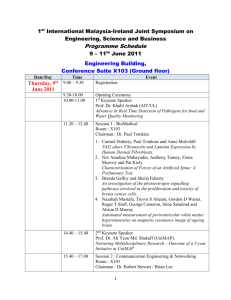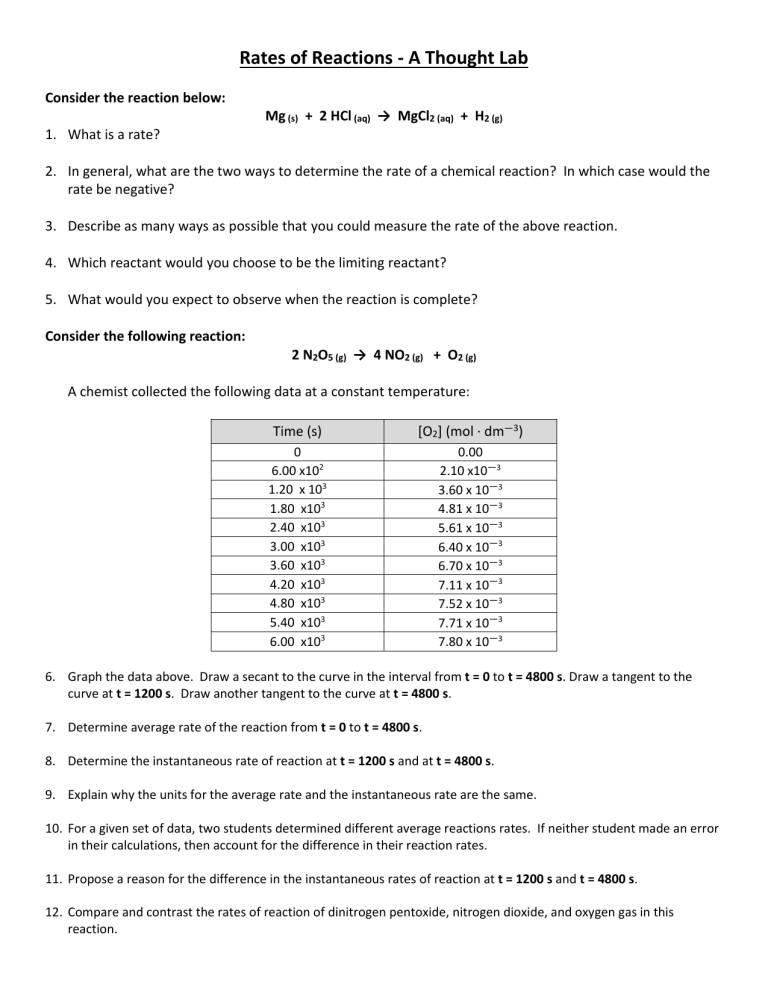
Rates of Reactions - A Thought Lab Consider the reaction below: Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2 (g) 1. What is a rate? 2. In general, what are the two ways to determine the rate of a chemical reaction? In which case would the rate be negative? 3. Describe as many ways as possible that you could measure the rate of the above reaction. 4. Which reactant would you choose to be the limiting reactant? 5. What would you expect to observe when the reaction is complete? Consider the following reaction: 2 N2O5 (g) → 4 NO2 (g) + O2 (g) A chemist collected the following data at a constant temperature: Time (s) [O2] (mol ∙ dm—3) 0 6.00 x102 1.20 x 103 1.80 x103 2.40 x103 3.00 x103 3.60 x103 4.20 x103 4.80 x103 5.40 x103 6.00 x103 0.00 2.10 x10—3 3.60 x 10—3 4.81 x 10—3 5.61 x 10—3 6.40 x 10—3 6.70 x 10—3 7.11 x 10—3 7.52 x 10—3 7.71 x 10—3 7.80 x 10—3 6. Graph the data above. Draw a secant to the curve in the interval from t = 0 to t = 4800 s. Draw a tangent to the curve at t = 1200 s. Draw another tangent to the curve at t = 4800 s. 7. Determine average rate of the reaction from t = 0 to t = 4800 s. 8. Determine the instantaneous rate of reaction at t = 1200 s and at t = 4800 s. 9. Explain why the units for the average rate and the instantaneous rate are the same. 10. For a given set of data, two students determined different average reactions rates. If neither student made an error in their calculations, then account for the difference in their reaction rates. 11. Propose a reason for the difference in the instantaneous rates of reaction at t = 1200 s and t = 4800 s. 12. Compare and contrast the rates of reaction of dinitrogen pentoxide, nitrogen dioxide, and oxygen gas in this reaction.