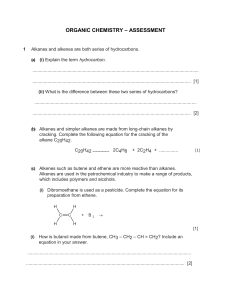
CHEMISTRY Matter and Change Chapter 22: Hydrocarbons Table Of Contents Section 22.1 Introduction to Hydrocarbons Section 22.2 Alkanes Section 22.3 Alkenes and Alkynes Section 22.4 Hydrocarbon Isomers Section 22.5 Aromatic Hydrocarbons Click a hyperlink to view the corresponding slides. Exit Introduction to Hydrocarbons • Explain the terms organic compound and organic chemistry. • Identify hydrocarbons and the models used to represent them. • Distinguish between saturated and unsaturated hydrocarbons. • Describe where hydrocarbons are obtained and how they are separated. microorganism: a tiny organism, such as a bacterium or a protozoan, that cannot be seen without a microscope Introduction to Hydrocarbons organic compound unsaturated hydrocarbon hydrocarbon fractional distillation saturated hydrocarbon cracking Hydrocarbons are carbon-containing organic compounds that provide a source of energy and raw materials. Introduction to Hydrocarbons Organic Compounds • Chemists in the early 19th century knew living things produced a variety of carbon compounds, called “organic” compounds. • They were not able to synthesize them in the lab and labeled them as mysterious. • Wöhler was the first to produce an organic compound in the lab. Introduction to Hydrocarbons Organic Compounds (cont.) • Organic compound is applied to all carbon-containing compounds with the primary exceptions of carbon oxides, carbides, and carbonates, which are considered inorganic. • In organic compounds, carbon nearly always shares its electrons and forms four covalent bonds. Introduction to Hydrocarbons Organic Compounds (cont.) • Carbon atoms are bonded to hydrogen atoms or other elements near carbon on the periodic table. • Carbon atoms also bond to other carbon atoms and form long chains. Introduction to Hydrocarbons Hydrocarbons • The simplest organic compounds are hydrocarbons, which consist of only the elements carbon and hydrogen. • There are thousands of hydrocarbons. Introduction to Hydrocarbons Hydrocarbons (cont.) • Carbon atoms bond to each other by single, double, and triple bonds. • Saturated hydrocarbons contain only single bonds. • Unsaturated hydrocarbons contain at least one double or triple bond. Introduction to Hydrocarbons Refining Hydrocarbons • M any hydrocarbons are obtained from the fossil fuel petroleum. • Petroleum, crude oil, is a complex mixture containing more than a thousand different compounds. In its raw form it is not very useful to humans, it is more useful when separated into simpler components or fractions. Introduction to Hydrocarbons Refining Hydrocarbons (cont.) • Fractional distillation involves boiling petroleum and collecting each group of components as they condense at different temperatures. Introduction to Hydrocarbons Refining Hydrocarbons (cont.) • Fractional distillation towers do not yield fractions in proportion to demand. • Heavier fractions are converted to gasoline or other lighter fractions by a process called cracking. – cracking is done in the absence of oxygen and in the presence of a catalyst. – In addition to converting heavier fractions to gasoline, cracking also produces starting materials for the synthesis of many different products including plastics, films and synthetic fibers. Introduction to Hydrocarbons Refining Hydrocarbons (cont.) • Gasoline is not a pure substance. • Most molecules have 5 to 12 carbons. • Gasoline is modified by adjusting its composition to improve performance, resulting in the octane rating system. Section Check Petroleum is separated into usable parts by boiling and condensing each component in a process called ____. A. cracking B. fractional distillation C. saturation D. bonding Section Check What is a hydrocarbon that contains only single bonds called? A. unsaturated B. organic C. saturated D. fully bonded Alkanes • Name alkanes by examining their structures. • Draw the structure of an alkane when given its name. • Describe the properties of alkanes. IUPAC (International Union of Pure and Applied Chemistry): an international group that aids communication between chemists by setting rules and standards in areas such as chemical nomenclature, terminology, and standardized methods Alkanes alkane homologous series parent chain substituent group cyclic hydrocarbon cycloalkane Alkanes are hydrocarbons that contain only single bonds. Alkanes Straight-Chain Alkanes • Alkanes are hydrocarbons that have only single bonds between atoms. Alkanes Straight-Chain Alkanes (cont.) • The names of alkanes end in –ane. • Prefixes are derived from Greek numbers. • A series of compounds that differ from one another by a repeating unit is called a homologous series. – For alkanes, the relationship between the numbers of carbon and hydrogen atoms can be expressed as: CnH2n + 2 Alkanes Branched-Chain Alkanes • Carbon atoms can bond to one, two, three, or four other carbon atoms making a variety of chains possible. Alkanes Branched-Chain Alkanes (cont.) • Straight chains and branched chains can have the same molecular formula. • Order and arrangement of atoms in organic compounds determine their identities. • When naming branched chain alkanes, the longest continuous chain of carbon atoms is called the parent chain. • All the side branches are known as substituent groups. Alkanes Branched-Chain Alkanes (cont.) Alkanes Branched-Chain Alkanes (cont.) • Naming branched-chain alkanes − Count the number of carbon atoms in the longest continuous chain. − Number each carbon in the parent chain, starting with the carbon closest to the substituent group. This gives all the substituent groups the lowest position numbers possible. − Name each alkyl group substituent. − If the same alkyl group appears more than once as a branch on the parent structure, use a prefix to indicate how many times it appears. Alkanes Branched-Chain Alkanes (cont.) • Naming branched-chain alkanes − When different alkyl groups are attached to the same parent chain, place their names in alphabetical order. − Write the entire name, using hyphens to separate numbers from words and commas to separate numbers. Alkanes Cycloalkanes • An organic compound that contains a hydrocarbon ring is called a cyclic hydrocarbon. • Cyclic hydrocarbons with only single bonds are called cycloalkanes. • The relationship between numbers of carbon and hydrogen atoms in cycloalkanes can be expressed as: CnH2n Alkanes Cycloalkanes (cont.) • Naming substituted cycloalkanes is the same as straight-chains but with a few exceptions. − The ring is always considered the parent chain. − Numbering starts on the carbon that is bonded to the substituent. − When more than one carbon has a substituent, number in the direction that gives the lowest possible numbers for the substituents. Alkanes Properties of Alkanes • Structure affects molecular properties. • Alkanes are not polar and are good solvents for other nonpolar molecules. Alkanes Properties of Alkanes (cont.) • Physical properties of alkanes − Compared to water, methane boils and melts at lower temperatures. − Methane molecules have little intermolecular attraction compared to water. (Methane molecules are nonpolar and do not form hydrogen bonds). Alkanes Properties of Alkanes (cont.) • Chemical properties of alkanes − Alkanes have low reactivity because they are nonpolar and have no charge, and because they have strong single bonds between carbon atoms. Section Check What is a cyclic alkane with no substituents groups and 6 carbon atoms? A. heptane B. cycloheptane C. cyclohexane D. cyclobutane Section Check Alkanes contain how many double bonds? A. greater than 2 B. 2 C. 1 D. 0 Alkenes and Alkynes • Compare the properties of alkenes and alkynes with those of alkanes. • Describe the molecular structures of alkenes and alkynes. • Name an alkene or alkyne by examining its structure. • Draw the structure of an alkene or alkyne by analyzing its name. hormone: chemical produced in one part of an organism and transported to another part, where it causes a physiological change Alkenes and Alkynes alkene alkyne Alkenes are hydrocarbons that contain at least one double bond, and alkynes are hydrocarbons that contain at least one triple bond. Alkenes and Alkynes Alkenes • Unsaturated hydrocarbons that contain at least one or more double covalent bonds between carbon atoms are called alkenes. Alkenes and Alkynes Alkenes (cont.) • Alkenes are named in much the same way as alkanes. • Alkenes end in –ene. • When four or more carbon atoms are present, specify the location of the double bond. • Note: Structure 3 is not 3Butene because it is identical to structure 1. Alkenes and Alkynes Alkenes (cont.) • When naming branched-chain alkenes, follow the same rules as for alkanes, with two exceptions. − The parent chain is always the longest chain that contains double bond, whether it is the longest chain or not. − The position of the double bond, not the branches, determine the numbering. − Use a prefix to designate the number of double bonds. Alkenes and Alkynes Alkenes (cont.) • Alkenes are nonpolar and have low solubility in water. • Alkenes are more reactive than alkanes because the double bond increases electron density between the two carbon atoms, providing a good site for chemical reactivity. Alkenes and Alkynes Alkynes • Unsaturated hydrocarbons that contain one or more triple bonds between carbon atoms are called alkynes. Alkenes and Alkynes Alkynes (cont.) • Straight-chain and branched-chain alkynes are named in the same way as alkenes, except the ending is –yne. Alkenes and Alkynes Alkynes (cont.) • Alkynes have physical and chemical properties similar to alkenes but are generally more reactive because the triple bonds cause even larger electron densities than double bonds. Section Check Which of the following is generally the most reactive? A. cycloalkanes B. alkanes C. alkenes D. alkynes Section Check What is the name of a straight-chain hydrocarbon with six carbon atoms and a triple bond between the second and third carbon atoms? A. 2-hexene B. 3-hexene C. 2-hexyne D. 3-hexyne Hydrocarbon Isomers • Distinguish between the two main categories of isomers—structural isomers and stereoisomers. • Differentiate between geometric isomers with cisand transprefixes. • Describe the structural variation in molecules that results in optical isomers. electromagnetic radiation: transverse waves that carry energy through empty space Hydrocarbon Isomers isomer chirality structural isomer asymmetric carbon stereoisomer optical isomer geometric isomer optical rotation Some hydrocarbons have the same molecular formula but have different molecular structures. Hydrocarbon Isomers Structural Isomers • Isomers are two or more compounds that have the same molecular formula but different molecular structures. • Structural isomers have the same chemical formula but their atoms are bonded in different arrangements. Hydrocarbon Isomers Structural Isomers (cont.) • There are two main classes of isomers: structural isomers and stereoisomers. • Structural isomers have different physical and chemical properties. • The structure of a substance determines properties. Hydrocarbon Isomers Stereoisomers • Stereoisomers are isomers in which all atoms are bonded in the same order but are arranged differently in space. Hydrocarbon Isomers Stereoisomers (cont.) • Geometric isomers result from different arrangements of groups around a double bond. • Cis means on the same side, and trans means across from. • Geometric isomers have different physical and chemical properties. Hydrocarbon Isomers Stereoisomers (cont.) Hydrocarbon Isomers Chirality • Louis Pasteur discovered two forms of crystallized tartaric acid. • The forms were mirror images of each other called right and left-handed forms. • The property in which a molecule exists in right and left-handed forms is called chirality. Hydrocarbon Isomers Optical Isomers • Chirality occurs whenever a compound contains an asymmetric carbon. • Asymmetric carbon is a carbon atom that has four different atoms or groups attached to it. • The four groups can be arranged in two different ways. Hydrocarbon Isomers Optical Isomers (cont.) • Isomers that result from different arrangements of four different groups around a carbon atom represent the other class of stereoisomers, called optical isomers. • Optical isomers have the same physical and chemical properties except in chemical reactions where chirality is important. Hydrocarbon Isomers Optical Isomers (cont.) • When polarized light passes through a solution containing an optical isomer, the plane of polarization is rotated to the right by the d-isomer and to the left by the lisomer, producing and effect called optical rotation. Hydrocarbon Isomers Optical Isomers (cont.) Section Check What are two molecules with the same formula but different structures called? A. isotopes B. chiral C. isomers D. asymmetric Section Check Which type of substances have the same physical and chemical properties but produce different optical rotations? A. isomers B. geometric isomers C. isotopes D. optical isomers Aromatic Hydrocarbons • Compare and contrast the properties of aromatic and aliphatic hydrocarbons. • Explain what a carcinogen is, and list some examples. hybrid orbitals: equivalent atomic orbitals that form during bonding by the rearrangement of valence electrons aromatic compound aliphatic compound Aromatic hydrocarbons are unusually stable compounds with ring structures in which electrons are shared by many atoms. Aromatic Hydrocarbons The Structure of Benzene • Even by the middle of the 19th century, hydrocarbon ring structures remained unknown. • Michael Faraday first isolated benzene in 1825. • Chemists knew the formula was C6H6 and proposed several possible models which would have all be very reactive, but benzene is unusually stable. Aromatic Hydrocarbons The Structure of Benzene (cont.) • German chemist Friederich Kekulé claimed to have had a dream in which he realized the flat, hexagonal shape ring structure for benzene. • Kekulé’s structure explained some of benzene's properties, but not its lack of reactivity. Aromatic Hydrocarbons The Structure of Benzene (cont.) • Linus Pauling’s hybrid orbital theory explained benzene’s lack of reactivity. • The double bonds in benzene are not fixed, but rather the electrons are delocalized and shared among all six carbon atoms. Aromatic Hydrocarbons Aromatic Compounds • Organic compounds that contain benzene rings as part of their structure are called aromatic compounds. • Aromatic was originally used because many benzene related compounds were found in pleasant smelling oils that came from plants and plant parts. Aromatic Hydrocarbons Aromatic Compounds (cont.) • Aliphatic compounds are the alkane, alkene, and alkyne hydrocarbons, coming from the Greek word for fat because they were obtained by heating animal fat. Aromatic Hydrocarbons Aromatic Compounds (cont.) • Substituted benzene compounds are named in the same way as cyclic alkanes. Aromatic Hydrocarbons Aromatic Compounds (cont.) • Many aromatic compounds were commonly used as industrial and laboratory solvents. • Health risks linked to aromatics include respiratory ailments, liver problems, and damage to the nervous system. • Some aromatic compounds cause cancer. Section Check Aliphatic compounds were originally obtained from ____. A. fossil fuels B. animal fats C. plant oils D. minerals Section Check Which is NOT true of benzene? A. It is an aromatic compound. B. It has a flat hexagonal shape. C. The double bonds make it unstable. D. It has delocalized electrons. Hydrocarbons Resources Chemistry Online Study Guide Chapter Assessment Standardized Test Practice Introduction to Hydrocarbons Study Guide Key Concepts • Organic compounds contain the element carbon, which is able to form straight chains and branched chains. • Hydrocarbons are organic substances composed of carbon and hydrogen. • The major sources of hydrocarbons are petroleum and natural gas. • Petroleum can be separated into components by the process of fractional distillation. Alkanes Study Guide Key Concepts • Alkanes contain only single bonds between carbon atoms. • Alkanes and other organic compounds are best represented by structural formulas and can be named using systematic rules determined by the International Union of Pure and Applied Chemistry (IUPAC). • Alkanes that contain hydrocarbon rings are called cyclic alkanes. Alkenes and Alkynes Study Guide Key Concepts • Alkenes and alkynes are hydrocarbons that contain at least one double or triple bond, respectively. • Alkenes and alkynes are nonpolar compounds with greater reactivity than alkanes but with other properties similar to those of alkanes. Hydrocarbon Isomers Study Guide Key Concepts • Isomers are two or more compounds with the same molecular formula but different molecular structures. • Structural isomers differ in the order in which atoms are bonded to each other. • Stereoisomers have all atoms bonded in the same order but arranged differently in space. Aromatic Hydrocarbons Study Guide Key Concepts • Aromatic hydrocarbons contain benzene rings as part of their molecular structures. • The electrons in aromatic hydrocarbons are shared evenly over the entire benzene ring. Hydrocarbons Chapter Assessment Organic compounds must contain which elements? A. carbon B. oxygen C. hydrogen D. nitrogen Hydrocarbons Chapter Assessment Cyclohexane contains how many hydrogen atoms? A. 6 B. 12 C. 14 D. 20 Hydrocarbons Chapter Assessment Which of the following has the greatest reactivity? A. benzene B. hexane C. hexene D. hexyne Hydrocarbons Chapter Assessment A carbon bonded to four different groups is ____. A. cyclic B. aromatic C. asymmetric D. left-handed Hydrocarbons Chapter Assessment Where were aromatic compounds originally obtained from? A. fossil fuels B. plant oils, plant parts, and spices C. animal fats D. minerals Hydrocarbons Standardized Test Practice Molecules that have the same formula and the atoms are bonded in the same order but are arranged differently in space, and have different properties are ____. A. structural isomers B. geometric isomers C. optical isomer D. sterioisotopes Hydrocarbons Standardized Test Practice If n is the number of carbon atoms in a hydrocarbon, what is the general formula of a cyclic alkane? A. CnHn+2 B. CnH2n+2 C. CnHn D. CnH2n Hydrocarbons Standardized Test Practice Which does NOT describe what happens as a liquid freezes? A. The temperature of the system is increased. B. Energy is released by the system. C. The liquid is entering the solid phase. D. The molecules begin to form a lattice. Hydrocarbons Standardized Test Practice What is a series of compounds that differ from one another by a repeating unit called? A. heterogeneous series B. homologous series C. straight-chain series D. branched-chain series Hydrocarbons Standardized Test Practice What structural characteristic do all aromatic compounds share? A. They are composed of cyclohexane. B. They have a triple bond. C. They contain a benzene ring. D. They contain a cyclic alkane. End of Custom Shows This slide is intentionally blank.