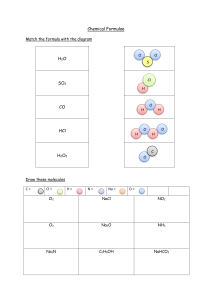
Chemistry homework ⇌ 2. a) N2 + 3H2 2NH3 with Fe (iron) uses as catalyst b) 2NH3(g) + H2SO4(aq) ----- (NH4)2SO4(aq) c) N , NH4NO3 = N/NH4NO3 = 2X14/14+4+14+16x3 = 28/80 = 0.35x100 = 35% 3.a) Ammonia gas is used to produce fertilizers for soil, which is used by growing plants and helps in protein synthesis. since it helps with crop cultivation, ammonia is important in feeding the world. b) *one environmental problem is eutrophication * one health problem is stomach cancer. c) nitrogen oxide, NO2 reacts with sulfur dioxide molecule, SO2, and the product of the reaction are sulfur trioxide,SO3 and nitrogen oxide,NO . NO then further reacts with oxygen giving NO2 becoming regenerated, the same NO2 from the first reaction now reacts with the second molecule of SO2. Nitrogen oxide is a catalyst for oxidation of SO2. d) *CO is oxidized to CO2 , C +2 to C +4 *NO is reduced to N2 , N +2 To zero