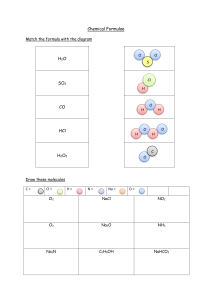
Name _________________ class ________ total 20 points 1. Assign oxidation number’s to chlorine from following compounds: [1 point] HClO; HClO3 ; KCl; HClO4 2. Circle the acidic oxides from following compounds: [1 point] SO2 ; K2O; Cl2O7; MgO; NO2 3. Acidic rain may be formed from following compounds: [1 point] NO2 ; SO2; FeO; CO2; PbO 4. From following [1*5 = 5 points] A: NH3 B: NaNO3 C: N2 D: HNO3 E: NO2 1) acidic oxide ____ 2) nonpolar covalent bonding ____ 3) ionic bonding ____ 4) oxidation state of N is +5 ____ 5) gas with basic properties _____ 5. Complete the reaction [2*3 = 6 points] 1) HNO3 diluted + Cu → 2) HNO3 cons + Zn → 3) S → SO2 → X → H2SO4 → K2SO4 6. Calculate the mass of oxygen needed to burn 256 kg sulphur. [2 points] S + O2 → SO2 7. Calculate the mass of produced phosphate salt by following reaction, if the mass of potassium hydroxide is 560 kg. KOH + H3PO4 → K3PO4 + H2O [2 points] 8. How many mass of KNO3 is needed to fill 5 L of jar with oxygen? [2 points] Name _________________ class ________ total 20 points 1. Assign oxidation number’s to chlorine from following compounds: [1 point] HClO; HClO3 ; KCl; HClO4 2. Circle the acidic oxides from following compounds: [1 point] SO2 ; K2O; Cl2O7; MgO; NO2 3. Acidic rain may be formed from following compounds: [1 point] NO2 ; SO2; FeO; CO2; PbO 4. From following [1*5 = 5 points] A: NH3 B: NaNO3 C: N2 D: HNO3 E: NO2 1) acidic oxide ____ 2) nonpolar covalent bonding ____ 3) ionic bonding ____ 4) oxidation state of N is +5 ____ 5) gas with basic properties _____ 5. Complete the reaction [2*3 = 6 points] 1) HNO3 diluted + Cu → 2) HNO3 cons + Zn → 3) S → SO2 → X → H2SO4 → K2SO4 6. Calculate the mass of oxygen needed to burn 256 kg sulphur. [2 points] S + O2 → SO2 7. Calculate the mass of produced phosphate salt by following reaction, if the mass of potassium hydroxide is 560 kg. KOH + H3PO4 → K3PO4 + H2O [2 points] 8. How many mass of KNO3 is needed to fill 5 L of jar with oxygen? [2 points]