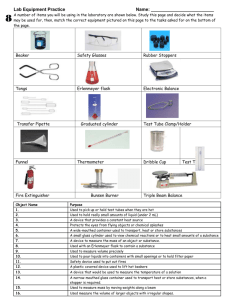
Specific Heat Capacity Challenges As hot oil is involved, you must wear safety goggles and lab coats Take time to think about and prepare for each challenge before starting any of them. Challenge 1: Using only the apparatus below, measure the specific heat capacity of the oil given. Apparatus: Access to water Oil 2 x beakers Tripod Gauze Heatproof mat Bunsen burner Stopwatch Thermometer Balance Challenge 2: Keep the hot oil as warm as possible for 10 minutes. Apparatus: Insulating materials Boiling tube Thermometer Hot oil Clamp stand Boss Clamp Stopwatch Heat proof gloves Funnel Method: Use your given share of the materials provided to insulate the boiling tube Use the clamp stand, boss, and clamp to hold the boiling tube Measure the temperature of the hot oil in the beaker Very carefully fill the boiling tube with hot oil As soon as you have done that, start the stopwatch After 10 minutes, measure the temperature of the oil in the boiling tube The winning team will be the one with the smallest percentage drop in temperature, found using this equation: % temperature change = initial temperature - final temperature × 100 initial temperature Challenge 3: Correctly predict the equilibrium temperature after a hot solid is added to water. Apparatus: Access to water Steel object 2 x beakers Tripod Gauze Heatproof mat Bunsen burner Thermometer Tongs Practical method: Using the balance, measure the mass of the steel object Place the steel object into a beaker with enough water to fully submerge the object Heat the water and solid to 100 degrees Celsius using the Bunsen burner Measure the mass of the second beaker Fill the second beaker with water and measure its temperature Measure the mass of the second beaker with the water and use this to find the mass of the water Gently add the steel object to the second beaker (using the tongs) and observe the maximum temperature reached by the water