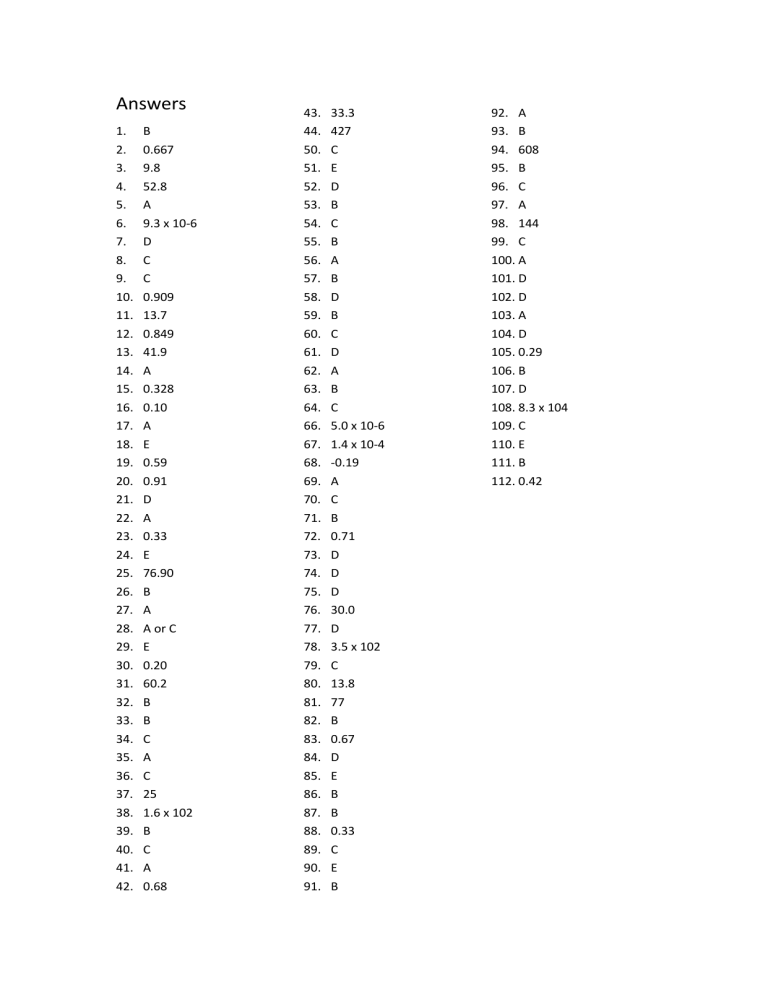
Answers 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. B 0.667 9.8 52.8 A 9.3 x 10-6 D C C 0.909 13.7 0.849 41.9 A 0.328 0.10 A E 0.59 0.91 D A 0.33 E 76.90 B A A or C E 0.20 60.2 B B C A C 25 1.6 x 102 B C A 0.68 43. 44. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. 33.3 427 C E D B C B A B D B C D A B C 5.0 x 10-6 1.4 x 10-4 -0.19 A C B 0.71 D D D 30.0 D 3.5 x 102 C 13.8 77 B 0.67 D E B B 0.33 C E B 92. A 93. B 94. 608 95. B 96. C 97. A 98. 144 99. C 100. A 101. D 102. D 103. A 104. D 105. 0.29 106. B 107. D 108. 8.3 x 104 109. C 110. E 111. B 112. 0.42 45. A "dipole" results from the partial charge separation that occurs when two atoms with significantly different electronegativities are covalently bonded. Since the electronegativities of two iodine atoms are identical, iodine will not have a permanent dipole. 46. Acetic acid contains the group "OH". This will allow acetic acid to hydrogen bond with the solvent, water, much more efficiently than ethyl acetate (which lacks any "OH" groups). The more favorable attractive forces between water and acetic acid will lead to greater solubility. 47. Hard water contains dissolved salts and minerals. Since freezing point is lowered as the number of solute particles in a solution is increased, hard water would be expected to have the lower freezing point. 48. 266 torr 49. The calibration curve was necessary because you needed to know how absorbance varied with concentration (of the dyes). Once you determined the relationship between absorbance and concentration, you could measure the absorbance of the mouthwash sample and find the concentration using the calibration curve. If you had simply measured the absorbance of the mouthwash, you would have no "scale" to use to obtain the concentration. Calibration curves are similar to a grading scale. If you know how "grade" varies with total points, you can determine your grade from your total points. However, without a grading scale (i.e., a calibration curve), you could not determine your grade, even if you knew your total points. 65. Water is a polar solvent. The fact that vitamins B and C are soluble in water suggests that these vitamins are much more polar than vitamins A, D, E and K. "Like dissolves like."