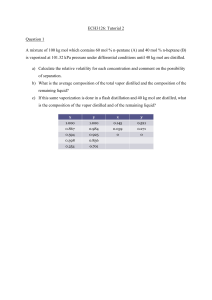
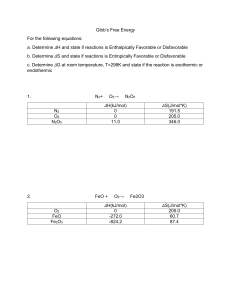
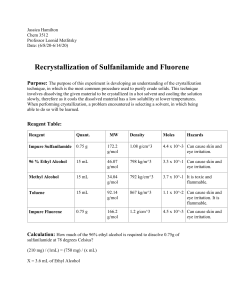
The relationship that exists between materials equilibrium moisture content (Xe) and water
activity (aw) during drying has been established by the Guggenheim–Anderson–de Boer (GAB)
model, represented as:
𝐶𝐾𝑎𝑤 𝑚0
𝑤 )(1−𝐾𝑎𝑤 +𝐶𝐾𝑎𝑤 )
𝐻
𝐶0 𝑒𝑥𝑝 (𝑅𝑇1 )
𝑋𝑒 = (1−𝐾𝑎
𝐶=
(1)
𝐻
{
𝐾 = 𝐾0 𝑒𝑥𝑝 (𝑅𝑇2 )
where the monolayer water content m0 = 0.06156 kg/kg, heats of sorption of water H1 = 24831
J/mol and H2 = -5118 J/mol, the molar gas constant R =8.314 J/mol/K, the absolute temperature
T = (52.6 + 273.15)K and the constants C0 = 0.001645 and K0 = 5.710.
Use appropriate programming language (Python, C or Java) and numerical approaches
(Bisection, Newton Raphson and Aitken’s Delta Square) to implement the numerical solution
for water activity (0<aw≤1), given that the equilibrium moisture content Xe = 0.133 kg/kg.
Hint: start by converting equation 1 to the form ax2 + bx + c = 0