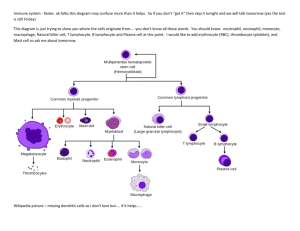
Lecture 13
CHAPTER4: COMPLEMENT SYSTEM
We'll talk about the experiment that led to the discovery of
complement:
In 1894, the scientist Bordet brought 2 test tube and put into each
tube, put an anti-serum (a serum containing antibodies) and then put
bacteria in each tube, but one tube was placed at a temperature of 37
° and the other at a higher temperature. Notice that in the tube
located at a temperature of 37 ° it occurred lysis of bacteria. As for
the tube at a high temperature, it did not get a lysis of bacteria, so
expect that there is something else that caused this (because the
only variable is bacteria)
So he concluded that there is another component in the serum, which
is heated- labile that complement the lytic function so he named it the
complement.
Since it is placed on both sides, the serum contains antibodies, and
on both sides there is bacteria and the temperature is the one that
differs.
Complement System: system of functionally linked proteins that
interact with one another in a highly regulated manner to provide
many of the effector functions of immunity and inflammation.
Characteristics of the complement system:
- It is part of the innate and adaptive immune response.
In the past, they put it in the innate, but since the word "antibody" was
mentioned, they put it under the adaptive
- Around 60% of the complement adult level is found in the fetus.
() هي من األشياء التي تبدأ العمل مبكرا.
- It consists of 16 complement proteins and 12 regulatory
proteins.
- It has 3 pathways: classical, alternative, lectin.
(lectin pathway is recently in immunology)
- It Is present in the serum as inactive state (zymogen) and
activation of the complements normally occur at certain
localized sites.
*Inactive: Regulation نوع من أنواع ال
zymogen: proteins that acquired proteolytic enzymatic
activity by the action of other protease by removing
inhibitory fragment and exposing the active sites.
Functions of the complement system: (briefly)
1- Lysing cells/ particles by forming pores in the cell surface causing
death by osmotic lysis. This is done by the terminal
component called the membrane attack complex (MAC).
Attack; pores وبعمل فيهاmembrane يهاجم ال
complex: complementsألنها تتكون من عدة
2- Help in the elimination of the Ag through opsonization (coating) ex:
complement 3b (C3b).
C3b very important opsonin phagocytic cells
(ex: macrophages, neutrophils)
Express receptors for these opsonins.
Opsonin: (complement proteins that can do opsonization are called
opsonins).
3- The phagocytic clearance of the immune complexes IC (Ag*Ab)
which could damage the tissues .
It is very important that Ab is associated with antigen and acts in
neutralization, but it must be removed. If it stays in the body, the I.C
will be deposited in various tissues and work on them and damage
the tissue the most is the kidney.
4- It aids in B cell activation
it helps the B cells to activate so this is one of the reasons for adding
complement to the adaptive
5- Triggering of inflammation by:
some complement fragments called chemotactic factors.
) )حفظex: C3a، C5a. –small fragment-
Chemotactic: promoting the migration of the inflammatory cells
especially the neutrophils into the site of inflammation .
*neutrophils : acute inflammatory response مشهورة في
and by some complement fragments called anaphylatoxins
(they stimulate mast cells) and basophils to release their
granular contents)
anaphylaxis .تساهم في عملية
ex: C3a, C4a, C5a .
From the book:(61-62-63)
The complement system was discovered at the end of the 19th
century as a heat-labile component of serum that augmented (or
‘complemented’) its bactericidal properties. Complement is now
known to comprise some 16 plasma proteins, together constituting
nearly 10% of the total serum proteins, and forming one of the major
immune defense systems of the body.
As we mentioned earlier in the experiment of serum protein electrophoresis,
separated into albumin and α, β, γ. γ globulin which contain antibodies. There are
little antibodies in beta, alpha, and among the proteins present in alpha and beta
are the complements, as they constitute 10% of the existing proteins Serum
In addition to acting as a key component of the innate immune
system, complement also interfaces with and enhances adaptive
immune responses. More than a dozen regulatory proteins are
present in plasma and on cells to control complement.
Complement activation pathways
The first activation pathway to be discovered, now termed the
classical pathway, is initiated by antibodies bound to the surface of
the target. Although an efficient means of activation, it requires an
adaptive immune response.
Fig 4.1: Role of complement in inflammation.
Fig 4.2:
The left part of fig is the classical pathway.
We note that the catalyst for it is the antigen
antibody complex, so it is placed within adaptive
for the presence of the word antibody. While on the
right part of the fig, the promoter for alternative and lectin
is microorganism. Note that C3 is shared between
the 3 pathways, and note how the small segment (a)
is removed and becomes (a) And (b) active and note
that the final output of 3 pathways is the MAC.
The alternative pathway, described in the 1950s, provides an
antibody-independent mechanism for complement activation on
pathogen surfaces.
The lectin pathway, the most recently described activation pathway,
also bypasses antibody to enable efficient activation on pathogens.
All three pathways – classical, alternative, and lectin pathways:
• involve the activation of C3, which is the most abundant and most
important of the complement proteins;
• comprise a proteolytic cascade in which complexes of complement
proteins create enzymes that cleave other complement proteins in an
ordered manner to create new enzymes, thereby amplifying and
perpetuating the activation cascade.
All activation pathways converge on a common terminal pathway – a
non-enzymatic system for causing membrane disruption and lytic
killing of pathogens.
The small chemotactic and proinflammatory fragments C3a
and C5a;
The large opsonic fragments C3b and C4b; and
The lytic membrane attack complex (MAC).
Fig4.3: Reference
from book (page.63)
-The classical pathway links to the adaptive immune
system
The classical pathway is activated by antibody bound to antigen
and requires Ca+2.
Ca
+2
يعتمد أيضا علىperforin - :للتذكير
Only surface-bound IgG and IgM antibodies can activate complement,
and they do so via the classical pathway. Surface binding is the key:
• IgM is the most efficient (because it is pentamer) activator, but
unbound (free) IgM in plasma does not activate complement.
• among
IgG subclasses, IgG1 and IgG3 are strong complement
activators, whereas IgG4 does not activate because it is unable to
bind the first component of the classical pathway.
Q. What occurs when IgM binds to the surface of a bacterium that
allows it to activate complement?
A: - A transition occurs from a flat planar molecule to a staple form, which exposes
binding sites for the first component of the complement system, C1
Fig4.4:
The first component of the pathway, C1, is a complex molecule
comprising a large, 6-headed recognition unit termed C1q and two
molecules each of C1r and C1s, the enzymatic units of the complex
(Fig. 4.4). Assembly of the C1 complex is Ca++-dependent, and the
classical pathway is therefore inactive if Ca++ ions are absent.
fig4.4(edition 9) 4.5 (edition8)
C1 activation occurs only when several of the head groups
of C1q are bound to antibody:
C1q in the C1 complex binds through its globular head groups to the Fc regions
of the immobilized (Ag.Ab) antibody .A single surface bound IgM can bind
multiple head groups and activate C1 ,but for IgG effective C1q binding depends
on the formation of hexametric IgG complex on the surface . Binding causes
changes in shape of C1q that
trigger autocatalytic activation of the enzymatic unit C1r.
Activated C1r then cleaves an adjacent C1s at a single site in the protein to
activate it.
Fig4.3(edition 9) (4.4 edition 8):..... مهم جدا جدا جدا جدا جدا جدا جدا
Ca
Mg
Mg
+
+
C5convertase
C4b2a3b
MAC complex
C1s enzyme cleaves C4 and C2
)28-29( *شرح بالعربي بالرابط أسفل الصفحة صفحات
The C1s enzyme has two substrates – C4 and C2 – which are the next two
proteins in the classical pathway sequence. (Note that the complement
components were named chronologically, according to the order of their
discovery, rather than according to their position in the reaction.)
C1s cleaves the abundant plasma protein C4 at a single site in the molecule:
• releasing a small fragment, C4a.
• exposing a labile thioester group in the large fragment C4b. Through the highly
reactive thioester, C4b becomes covalently linked to the activating surface C4b
binds the next component, C2, in a Mg++-dependent complex and presents it for
cleavage by C1s in an adjacent C1 complex:
• the fragment C2b is released.
• C2a remains associated with C4b on the surface. C4b2a is the classical
pathway C3 convertase The complex of C4b and C2a (termed C4b2a – the
classical pathway C3 convertase) is the next activation enzyme. C2a in the
C4b2a complex cleaves C3, the most abundant of the complement proteins:
• releasing a small fragment, C3a.
• exposing a labile thioester group in the large fragment C3b. As described
above for C4b, C3b covalently binds the activating surface. C4b2a3b is +0.
C5 convertase Some of the C3b formed will bind directly to C4b2a, and the
trimolecular complex formed, C4b2a3b (the classical pathway C5 convertase),
can bind C5 and present it for cleavage by C2a
a small fragment, C5a, is released and
1
• The larger fragment C5b associates with C6 and C7, which can then
bind to plasma membranes. The complex of C5b67 assembles C8 and a
number of molecules of C9 to form a membrane attack complex (MAC).
classical pathway تم بحمد هللا
1
https://drive.google.com/file/d/1NeG1Ktnd90gZwIQTTLsKRc9ktjgKGZBi/view?fbclid=IwAR3k_4djJp3yIWD
ffxpsQ3vjk4NGXTXYUDaiWt-L5sUSge5K5xerTO7FaNY
Lectin pathway:
Lectin: proteins bound to carbohydrates.
The lectin pathway is similar to the classical pathway except the initial step
(activation).
The lectin pathway is activated by the MBL (mannose binding lectin)
which binds to the mannose residue on the surface of the microorganism.
MBL: an acute phase protein produced in the inflammatory responses. It
is similar in structure and function to C1.
وهو عبارة عن بروتين يتواجد في جسمنا في حاالت,MBL هوLectin لactivation الذي يعملC1 (بدل
لactivation يرتبط به ويبدأmannose عليةmicroorganism عندما يجد امامه, inflammation
)lactin Pw
Fig.4.3 نرجع ل
(بفضل ان تكتبوا بجانبه في الصورةCa2+ يحتاج الى عنصرMBL،lectin Pw activation الحظ مستطيل
في التركيب والوظيفة ) ال نريد التفاصيل األخرى في المربعC1 فهو أصال متشابهة ل، Ca2+
بوجودC4b علىC2 ثم يأتيC4a,C4a الىC4 يحطمMBL ، ) تبدأ مباشرة بأنficolins/MASP-2(
C4b2a الناتج, يكمل المسارC2a , C2a<C2b الىC2 , MBL ثم يحطمC4b2 <<<< Mg2+
وهكذا الى اخر المسار...... ,C3a ,C3b الىC3 الذي يحطمC3 convertase =
Froom book page 65
The alternative and lectin pathways provide antibodyindependent ‘innate’ immunity
)Ab (ال يحتاج لوجود
* The lectin pathway is activated by microbial
carbohydrates.
The lectin pathway differs from the classical pathway only in the initial
recognition and activation steps. Indeed, it can be argued that the lectin
pathway should not be considered a separate pathway, but rather a route
for classical pathway activation that bypasses the need for antibody.
The C1 complex is replaced by a structurally similar molecule
(MBl)
lectin pathway تم بحمد هللا
122+11 الشرح بالعربي موجود بالرابط اسفل الصفحة بصفحة
The alternative pathway is activated by the presence of the
microorganism only, it starts with the cleavage of C3 (we don’t need
C2, C4 & C1 in this pathway, which means that if the child has
abnormality in them the alternative pathway is not affected but the
classical & lectin pathways will be lost) into C3a & C3b:
>> C3a will defuse.
>> C3b will complete the pathway. There is a protein in our bodies
referred to as B protein in the fig. will bind to C3b to give C3bB in the
presence of Mg+2 A protease found in serum in very little amounts in our
bodies referred to as D in the fig. will interact with C3bB to cleave protein
B into Ba & Bb:
>> Ba will defuse.
>>> Bb will continue the pathway in the C3bBb (C3 convertase), to cleave
another C3 into C3a & C3b:
>>> C3a will defuse.
>>> C3b will complete the pathway which will join C3bBb.
This will result in the formation of C3bBb3b (C(3b)2Bb) C5 convertase
which will cleave C5 into C5a & C5b.
>>> C5a will defuse away >>>
>>>C5b will complete the pathway.
Then C6 & C7 will act with C5b to give C5b67 complex, then both C8 &
C9 will act with C5b67 to give C5b6789 (C5b-9), which is called
membrane attack complex (MAC).
Alternative pathway activation is accelerated by microbial
surfaces and requires Mg++.
The alternative pathway of complement activation also provides antibodyindependent activation of complement on pathogen surfaces. This
pathway is an constant state of low-level activation.
The C3bBb complex is the C3 convertase of the alternative pathway.
alternative pathway تم بحمد هللا
2
https://drive.google.com/file/d/1NeG1Ktnd90gZwIQTTLsKRc9ktjgKGZBi/view?fbclid=IwAR3k_4djJp3yIWD
ffxpsQ3vjk4NGXTXYUDaiWt-L5sUSge5K5xerTO7FaNY
Lecture 15
Regulation of the complement cascade / pathway /mechanism.
Uncontrolled activation of complements can lead to consumption of
the complement proteins and can lead to formation of MAC on selftissues and excessive generation of inflammatory mediators.
This does not happen normally because
1- Activation of the complement cascade doesn’t occur
spontaneously.
2- And because of the presence of the regulators (12) either as
soluble form in plasma or membrane-bound.
Examples of classical and lectin pathway regulators:
امثلة نفسهم بالجهتين---- نفس المسار ما عدا اول الخطوةlectin وclassical بما ان
-
Complement 1 inhibitor or C1 and MBL inhibitor.
)(يمنع المسار من اولهMBL وC1 يعمل منع ل
C1 and حديثا اصبح اسمهlectin هذا االسم كان سابقا لكن بعد اكتشافC1 inhibitor (
) في التركيب والوظيفةC1 مشابه لMBL ف،MBL inhibitor
C4b binding protein –soluble plasma- preventing C2 from
binding.
Complement receptor type 1 CR1 –membrane bound- preventing
C2 from binding.
Decay accelerating factor DAF it acts on C4b.
Anaphylatoxins inactivators by removing the terminal arginine
residue from C3b, C4a, C5a (anaphylatoxins) to inactive them.
3-pathwy بشتغل على ال
1
Examples of alternative pathway regulators:
- Factor H /protein H – it competes factor B from binding to C3b
{soluble}
)C3Bh وينتجC3b معH يرتبطC3Bb (بدل ما ينتج
- Complement receptor type 1 – can bind to C3b.
Membrane-bound
- Decay accelerating factor DAF – it decays C3b.
- Factor I – it cleaves C3b.
Examples of MAC formation regulation:
)< <وضعناهم ضمن نفس المجموعة3 pathways (بما ان المراحل النهائية متشابهة في
o Factor S/protein S it binds C5b67 preventing C8 from binding.
o Homologous restriction factor prevents C9 from binding.
{soluble}
o CD59 {membrane bound} prevents C9 from binding to C8.
From book (66-CH-4)
Complement protection systems:
Control of the complement system is required to prevent the consumption of
components through unregulated amplification and to protect the host. Complement
activation poses a potential threat to host cells, because it could lead to cell opsonization
or even lysis. To defend against this threat a family of regulators has evolved alongside
the complement system to prevent uncontrolled activation and protect cells from attack.
C1 inhibitor controls the classical and lectin pathways.
2
Table 4.2:
لplasma موجودين فيfactor H,C4 binding protein الحظ ان،ليس مطلوب التفاصيل فيها
) membrane- bound( Different cell موجودين علىDAF,CR1 والحظsoluble form
From book (page 68)
The membrane attack pathway
Activation of the pathway results in the formation of a transmembrane pore: The terminal or membrane attack pathway involves a distinctive set of events whereby a
group of five globular plasma proteins associate with one another and, in the process,
acquire membrane-binding and pore-forming capacity to form the membrane attack
complex (MAC).
The MAC is a transmembrane pore. Cleavage of C5 creates the nidus for MAC
assembly to begin. While still attached to the convertase enzyme, C5b binds first C6
then C7 from the plasma. Conformational changes occurring during assembly of this
trimolecular C5b67 complex.
Membrane-bound C5b67 recruits C8 from the plasma and finally multiple copies of C9
are incorporated in the complex to form the MAC.
3
The fully formed MAC creates a rigid pore in the membrane, the walls of which are
formed from multiple copies of C9 (up to 12), arranged like barrel staves around a
central cavity.
The MAC is Cleary visible in electron microscope images.
The pore:
• allowing the free flow of solutes and electrolytes across the cell membrane.
• because of the high internal osmotic pressure, causing the cell to swell and sometimes burst.
Regulation of the membrane attack pathway reduces the risk of ‘bystander’ damage to
adjacent cells.
)تعني بالصدفة موجودة في منطقة الحدث (الخاليا المجاورة غير متداخلة في العملية
(complement (حول عمليةdamage <<< فهذا يحمي المنطقة المجاورة منMAS عندما يحدث تنظيم ل
Fig4,8:-(edition 9(4.10 edition 8) مرور
4
Fig.4.9
MAC الجزء العلوي من الرسمة يوضح تكون
وهوregulators الجزء السفلي من الرسمة يتكلم عن احد
هو بروتين موجود على الخاليا عندما يأتيCD59 , CD59
يشبه الزنبرك) فال يتكونCD59( يبعدهC8 يرتبط معC9
.MHC
From book (page 69)
The membrane binding site in C5b67 is labile. If the complex does not
encounter a membrane within a fraction of a second after release from the
convertase, the site is lost.
Membrane receptors for complement products:
Receptors for fragments of C3 are widely distributed on different
leukocyte populations.
فسيعمل علىanaphylaxisn ويعمل ك,...neutrophils لجذبchemotactic يعمل لC3a
الذي يعمل, phagocytosis لتسهيل عمليةopsonin بعمل كC3b , mast cell, basophils
<<<< لذلك يجب ان يكون عليهمneutrophils , macrophage <<<< phagocytosis
) C3b لReceptor
5
)التي ذكرناها سابقا مع بعض التوضيحاتcomplement (النقاط القادمة من الكتاب هي وظائف
C5a and C3a are chemotactic for macrophages and polymorphs
Fig 4.10 (edition 9) (4.13 edition 8)
ويعمالن على إطالق محتوياتهاmast cell يعمالن علىC3a /C5a الحظ ان
-----------------------------------------------------------------------------
thC3a, C4a and C5a activate mast cells and basophils.
cytokines ،histamine <<<< اشهر محتوياتها
They are called anaphylatoxin
C3b and its breakdown product iC3b.
6
C3b and iC3b and C4b
are important opsonins:
Fig 4.11
CR <<<phagocytic cell ) التي حوطت البكتريا يوجد علىcomplement (C3b,iC3b, C4bالحظ البكتيريا و
.التي اخذناها في مادة الفيرستphagocytosis ثم تبدأ عمليةcomplement receptor)(
7
C3b disaggregates immune complexes and promotes their clearance.
Immune complexes containing antigens derived either from pathogens or from the
death of host cells form continuously in health and disease. Because they tend to grow
by aggregation and acquisition of more components, they can cause disease by
precipitating in capillary beds in the skin, kidney, and other organs, where they drive
inflammation.
Complement activation on the immune complex via the classical pathway efficiently
opsonizes the immune complex and helps prevent precipitation in tissues:
How can C3b get rid of I.C?
By 3 ways:
First: coating with C3b masks the foreign antigens in the core of the immune
complex, blocking further growth.
Second: coating with C3b disaggregates large immune complexes by disrupting
interactions between antigens and antibodies.
Third: C3b (and C4b) on immune complexes interacts with CR1 on erythrocytes,
taking the immune complex out of the plasma – the immune adherence
phenomenon.
C3b and C4b associate with Ag*Ab(I.C) then a receptor on the RBCs binds with them
taking them out from the plasma, this proses is known by immune adherence
phenomenon.
The MAC damages some bacteria and developed viruses:
Assembly of the MAC creates a pore that inserts into and through the lipid bilayer
-that’s why MHC is effective against G- bacteria (they have lipid bilayer) and not
effective against G+
→ Immune complexes with bound C3b are very efficient in primary activation B cells
8
fig4.12 complement plays important role in adaptive immunity
9
Biosynthesis of complement proteins:
Both hepatocytes and mononuclear phagocytes(macrophages) can synthesize most of
the complement proteins found in serum. The liver makes more quantity but
mononuclear phagocyte synthesis is significant at the site of infection.
(the liver is large so it gives good amounts, but the liver is fixed not like the mononuclear
phagocytes that are distributed in the body so it exists in the area of the infection)
C1 can be synthesized by various types of epithelial cells other than the hepatocytes.
Diseases related to the complement system:
It is related in two general ways:
First: deficiency of absence in any one of the protein components usually due to
abnormalities in gene structure. If regulatory proteins (12) are deficient or absent,
too much complement activation can occur at the wrong time or wrong site,
while if the absence or deficiency in any of the complement proteins (16), too little
complement activation and lack of complement mediated functions.
Second: normal function of the complements in response to abnormal stimuli
such as persistent microorganism ( )يكون في تحفيز طالما هو موجودor autoimmune
disease ()تتجاوب مع اشياء طبيعية على انها غريبة
From book: page 84 +85 +86
Complement deficiencies:
Genetic deficiencies of each of the complement components and many of the regulators
have been described and provide valuable ‘experiments of nature’ illustrating the
homeostatic roles of the complement. In general, complement deficiencies are rare,
though some deficiencies are much more common in some racial groups.
10
fig 4.13, complement system deficiencies
Not fig.4.13
Look in the top the name of the pathway and under it its stimulus.
classical begins with C1, C2 and C4, any deficiency in any of them causes a
disease called lupus-like disease ( like systemic lupus erythematosus SLE)
lectin is like classical except the last step, deficiency in MBL causes severe
recurrent bacterial infections.
any deficiency in factors B/D causes severe recurrent bacterial infections.
any deficiency in C3 causes severe recurrent bacterial infections.
any deficiency in C5, C6, C7, C8, C9 (that make MAC) causes recurrent
Neisserial infections (because MAC’s impact on G- is bigger).
11
Classical pathway deficiencies result in tissue inflammation:
Deficiency of any of the components of the classical pathway (C1, C4, C2) predisposes
to a condition that closely resembles the autoimmune disease systemic lupus
erythematosus SLE.
Deficiencies of MBL are associated with infection in infant:
At least 10% of the population have MBL levels below 0.1 mg/ml(not for memorizing)
and are considered to be MBL deficient. MBL deficiency is associated in infants with
increased suitability to bacterial infections. This tendency disappears as the individual
ages and the arms of immunity matures.
(in infants, Abs levels did not reach high enough, and the marginal zone still incomplete
so the immunity normally is low in his body, so if he has MBL deficiency his situation is
much worse, but it improves after that because Abs and marginal zone gets better
🙁اسف اإلنجليزية التعبانة
(the infant got better not for an improvement in the MBL, it’s for an improvement in other
arms of immunity)
Alternative pathway and C3 deficiencies are associated with bacterial infections.
Terminal pathway deficiencies predispose to Gram-negative bacterial infections:
Deficiencies of any of the terminal complement components (C5, C6, C7, C8 or C9)
predisposes to infections with Gram-negative bacteria, particularly those of the genus
Neisseria. This genus includes the meningococcal responsible for meningococcal
meningitis and the gonococci responsible for gonorrhea.
Q: why should these deficiencies be specifically associated with infection by gramnegative bacteria and not with all bacterial infections?
Answer: gram-negative bacteria have an outer phospholipid membrane, which may be
targeted by the lytic pathway. Gram-positive bacteria have a thick bacterial cell wall on
the outside.
12
:مهم
C1 inhibitory (regulatory protein) deficiency causes hereditary angioedema
autoantibodies against complement components, regulators and complexes also
cause disease.
(it is not always because of a deficiency, there may be autoantibodies against a specific
complement so it stops its function)
From book page 75
Hereditary angioneurosis edema (HAE) results from C1 inhibitor deficiency:
Patients have recurrent episodes of swelling of various parts of the body (angioedema).
When the edema involves the intestine, excruciating abdominal pain and cramps
result, with severe vomiting.
When the edema involves the upper airway, the patients may choke to death
from respiratory obstruction- angioedema of the upper airway therefore presents
a medical emergency, which requires rapid action to restore normal breathing.
fig 4.14 hereditary angioneurotic edema
Look how the person looks
13
Fig.4.15
14
Lecture 17
بالبداية رح نحكي عن معلومات من كتاب خارجيimmune inflammation رح نبلش في ال
... بالكتاب3 ثم نعود تشابتير
Mediators of Inflammation
During an inflammatory response, a variety of mediators are released by cells of the
innate and acquired immune systems. These mediators serve to trigger or enhance
specific aspects of the inflammatory response. They are released by tissue mast
cells, blood platelets, and a variety of leukocytes, including neutrophils,
monocytes/macrophages, eosinophils, basophils, and lymphocytes.
)inflammation (تقريبا جميع الخاليا تشارك في عملية ال
mediators of inflammation:(4 ) انواع
1. Chemokines
Chemokines are, a superfamily of small polypeptides, most of which contain
90-130 amino acid residues. They selectively, and often specifically,
control the adhesion, chemotaxis, and activation( ) وظائفهاof many types of
leukocyte populations and subpopulations. Consequently, they are major
regulators of leukocyte traffic.
A variety of lymphoid and no lymphoid tissues can produce chemokines, often
in connection with the initiation or progress of inflammation. However,
members of this group are also involved in the regulation of normal leukocyte
traffic into, out of, and within lymphoid tissues and organs.
→ Chemokines cause leukocytes to move into various tissue sites
by inducing the adherence of these cells to the vascular
endothelium. After migrating into tissues, leukocytes are attracted
toward high localized concentrations of chemokines.( الذي هو بمكان ال
inflammation (
Recent work has also shown that some chemokines are involved in the
development of nonlymphoid tissues, such as the heart.
→ The chemokines possess four conserved cysteine residues and can
be separated into two distinctive subgroups based on the position of
two of the four invariant cysteine residues.
1. C-C subgroup chemokines, in which the conserved cysteine's
are contiguous.
2. C-X-C subgroup chemokines, in which the conserved
cysteine's are separated by some other amino acid (X)
1
Chemokine action is mediated by receptors whose polypeptide chain
traverses the membrane seven times, Because of their snakelike
pattern of repeated penetration and emergence from the membrane,
they are called serpentine receptors. When they bind the appropriate
chemokine, these receptors activate large GTP-binding proteins, which
are heterotrimeric G proteins. This initiates signal transduction
processes that generate such potent second messengers as CAMP,
IP3, Ca, and activated small G proteins (Figure 15-8).
FIGURE 15-8 Chemokines signal through receptors coupled with large heterotrimeric G proteins. Binding of a chemokine to its receptor activates many
signal-transduction pathways, resulting in a variety of modifications in the
physiology of the target cell. If the signal-transduction pathway is not known or
incompletely worked out, dashed lines and question marks are used here to
represent probable pathways. Premack et al, 1996, Nature Medicine
2
Dramatic changes are effected by the chemokine-initiated activation of these
signal transduction pathways. Within seconds, the addition of an appropriate
chemokine to leukocytes causes abrupt and Extensive changes in shape, the
promotion of greater adhesiveness to endothelial walls/by a ctivation of
leukocyte integrin's, and the generation of microbicide oxygen radicals in
phagocytes. These signal transduction pathways promote other changes such
as the release of granular contents, ex: proteases in neutrophils and macrophages, histamine from basophils, and
cytotoxic proteins from eosinophils
3
A number (more than 50) of chemokines and multiple (14) chemokine
receptor have been described.
) IL-8 هائلة في الجسم الوحيد المطلوب هو اشهر واحد هوchemokines ( اعداد ال
2. Plasma Enzyme Mediators 4تتضمن
Plasma contains four interconnected mediator-producing systems:
a)
b)
c)
d)
the kinin system,
the clotting system,
the fibrinolytic System,
and the complement system.
With the exception of the complement system, these systems share
a common intermediate, as illustrated in (Figure 15-10). When
tissue damage occurs, these four systems are activated to form a
web of interacting systems that generate a number of mediators of
inflammation.
a) Kinin System
The kinin system is an enzymatic cascade that begins when a
plasma clotting factor, called Hageman factor, is activated
following tissue injury. The activated Hageman factor (12) then
activates prekalliktein to form kallikrein, which cleaves
kininogen to produce bradykinin (see Figure 15-10).
Inflammation mediator of kinin system.
This inflammatory mediator is a potent vasoactive basic peptide
that increases vascular permeability, causes vasodilation, induces pain, and induces contraction of smooth muscle.
Kallikrein also acts directly on the complement system by
cleaving C5 into C5a and C5b. The C5a complement component is an anaphylatoxin that induces mast cell degranu- la
tion, resulting in the release of a number of inflammatory
mediators from the mast cell.
b) Clotting System
Another enzymatic cascade that is triggered by damage to
blood vessels yields large quantities of thrombin. Thrombin acts
on soluble fibrinogen in tissue fluid or plasma to produce
insoluble strands of fibrin and fibrin peptides The insoluble
fibrin strands crisscross one another to form a clot, which
serves as a barrier to the spread of infection.
The clotting system is triggered very rapidly after tissue injury to
1-prevent
4
bleeding and limit the 2-spread of invading pathogens into the
bloodstream. The fibrin peptides act as Inflammatory mediators,
inducing increased vascular permeability and neutrophil
chemotaxis.
c) Fibrinolytic System
Removal of the fibrin clot from the injured tissue is achieved by
the fibrinolytic system. The end product of this pathway is the
enzyme plasmin which is formed by the conversion of
plasminogen. Plasmin, a potent proteolytic enzyme breaks
down fibrin dots into degradation products that are chemotactic
for neutrophils. Plasmin also contributes to the Iniammatory
response by activating the classical complement pathway.
classical complement PW. لactivation تعرفوا على شيء إضافي يعمل
) Ag-Ab) (الشيء األول هوplasmin وهو
d) Complement System
Activation of the complement system by both classical and
alternative pathways results in the formation of a number of
complement split products that serve as important me- diators of
inflammation. Binding of the anaplylatoxins (C3a, C4a, and C5a)
to receptors on the membrane of tissue mast cells induces
degranulation with release of histamine and other
5
pharmacologically active mediators. These mediators induce
smooth-muscle contraction and increases in vascular permeability.
C3a, C5a, and Csb67 act together as chemotactic.
3. Lipid Inflammatory Mediators
Following membrane perturbations, phospholipids in th membrane of
several cell types (e.g., macrophages, monocytes, neutrophils, and mast
cells) are degraded into arachidonic acid and lyso-platelet-activating factor
(Figure 15-11).
The latter is subsequently converted into platelet-activating factor (PAF)
This factor causes platelet activation and has many inflammatory effects,
including cosinophil chemotaxis and the activation and degranulation of
neutrophils and eosinophils
. Metabolism of arachidonic acid: - by the cyclooxygenase pathway
produces prostaglandins and thromboxane's Different prostaglandins
are produced by different cells: monocytes and macrophages produce
large quantities of PGE2 and PGF2; neutrophils produce moderate
amounts of PGE2; mast cells produce PGD2.
Prostaglandins have diverse physiological effects, including increased
vascular permeability increased vascular dilation, and induction of
neutrophil chemotaxis. The thromboxane's cause platelet aggregation and
constriction of blood vessels.
Arachidonic acid is also metabolized by the lipoxygenase pathway to yield
leukotrienes. There are four leukotrienes:
(LTB4, LTCA, LTD4, and LTE4.) Three of these (LTC4, LTD4, and LTEA)
together make up what was formerly called slow reacting substance of
anaphylaxis (SRS-A); these mediators induce smooth muscle contraction
LTB4 is a potent chemoattractant of neutrophils.
3 leukotrienes (LTC,LTD,LTE) مكون من
6
Figure 15 .11 The breakdown of membrane phospholipids
generate important mediators of inflammation, including
thromboxane, prostaglandins, leukotrienes and platelet activating
factor (PAF).
-----------------------------------------------------------------------------------
4. Cytokine Inflammatory Mediators
A number of cytokines play a significant role in the development of an
acute or chronic inflammatory response IL-1 IL-6 TNF-a, and many
chemokines exhibit redundant and pleiotropic effects that together
contribute to the inflammatory response.
Tumor necrosis factor
The Inflammatory Process
Inflammation is a physiologic response to a variety of stimuli such
as infections and tissue injury. In general, an acute inflammatory
response has a rapid onset and lasts a short while. Acute inflammation
is generally accompanied by a systemic response(ex- fever) known as
the acute-phase response, which is characterized by a rapid alteration
in the levels of several plasma proteins. In some diseases persistent
immune acti- vation can result in chronic inflammation, which often has
pathologic consequences.
Central Role df Neutrophils in Inflammation )الخلية األساسية في عملية
(Acute inflammation
In the early stages of an inflammatory response, the predominant cell
type infiltrating the tissue is the neutrophil.
Neutrophil infiltration into the tissue peaks within the first 6 h of an
inflammatory response. Neutrophil production in the bone marrow
7
increases to meet this need. A normal adult produces more than
1010neutrophils per day, but during a period of acute inflammation,
neutrophil production may increase as much as tenfold.
inflammation) في حاالت الneutrophils يحدث انتاج ل1030 عشرة اضعاف
The neutrophils leave the bone marrow and circulate within the blood.
In response to mediators of acute inflammation, vascular endothelial
cells increase their expression of E- and P-selectin. Thrombin and
histamine induce in- creased expression of P-selectin; cytokines such
as IL-1 or TNF-a induce increased expression of E-selectin. The
circulating neutrophils express mucins such as PSGL-1 or the tetra
saccharides silyl Lewis" and sialyl Lewis", which bind to E- and Pselectin.
8
Once in tissues, the activated neutrophils also express in- creased
levels of receptors for chemoattractant and therefore exhibit
chemotaxis, migrating up a gradient of the chemoattractant. Among the
inflammatory mediators that are chemotactic for neutrophils are several
chemokines complement split products (C3a, C5a, and C5b67),
fibrin peptides, prostaglandins, and leukotrienes.
The activating signal also stimulates metabolic pathways to a
respiratory burst, which produces reactive oxygen in- (ocl-) termediates
and reactive nitrogen intermediates. Release of some of these reactive
intermediates and the release of me- diators from neutrophil primary
and secondary granules (proteases, phospholipases, elastases, and
collagenases) play important role in killing various pathogens. These
substances also contribute to the tissue damage that can result from
an inflammatory response. The accumulation of dead cange cells
and microorganisms, together with accumulated Hud and various
proteins, makes up what is known as pus.
Acute Inflammatory Response
Infection or tissue injury induces a complex cascade of non- specific
events, known as the inflammatory response, that provides early
protection by restricting the tissue damage to the site of infection or
tissue injury. The acute inflammatory response involves both localized
and systemic responses.
Localized Response
The hallmarks of a localized acute inflammatory response, first
described aimost 2000 years ago, are swelling (tumor), redness
(ruber), heat (calor), pain (dolor), and loss of function. Within minutes
after tissue injury, there is an increase in vascular diameter
(vasodilation), resulting in an increase in the volume of blood in the
area and a reduction in the flow of blood. The increased blood volume
heats the tissue and causes it to redden. Vascular permeability also
increases, Treading to leakage of fluid from the blood vessels,
particularly at post capillary venules. This results in an accumulation
bon of fluid (edema) in the tissue and, in some instances, extravasation
of leukocytes, contributing to the swelling and redness in the area.
When fluid exudes from the bloodstream, a the kinin, clotting, and
fibrinolytic systems are activated (see Figure 15-10). Many of the
vascular changes that occur early ti in a local response are due to the
direct effects of plasma enzyme mediators such as bradykinin and
fibrin peptides, vessels to which induce vasodilation and increased
vascular perme- ability. Some of the vascular changes are due to the
indirect effects of the complement anaphylatoxins (C3a, C4a, and
C5a), which induce local mast-cell degranulation with release of
histamine.
9
Histamine is a potent mediator of inflammation, causing vasodilation
and smooth-muscle con- traction. The prostaglandins can 'also
contribute to the vasodilation and increased vascular permeability
associated with the acute inflammatory response.
Within a few hours of the onset of these vascular changes,
neutrophils adhere to the endothelial cells, and migrate out of the blood
into the tissue spaces (Figure 15-12). These neutrophils phagocytose
invading pathogens and release mediators that contribute to the
inflammatory response. Among the mediators are the macrophage
inuammatory proteins (MIP-1a and MIP-1B), chemokines that attract
macro- phages to the site of inflammation. Macrophages arrive about
5-6 hours after an inflammatory response begins. These macrophages
are activated cells that exhibit increased phagocytosis and increased
release of mediators and cytokines that contribute to the inflammatory
response. Activation tissue macrophages (IL-1, IL-6, and TNF-a) that
induce many of the localized and systemic changes observed in the
acute inflammatory response.
A local acute inflammatory response can occur without the overt
involvement of the immune system. Often, however, cytokines released
at the site of inflammation facilitate both the adherence of immunesystem cells to vascular endothelial cells and their inigration through
the vessel „wall into the tissue spaces.
The result is an influx of lymphocytes, neutrophils, monocytes,
eosinophils, basophils, and mast cells to the site of tissue damage,
where these cells participate in clearance of the antigen and healing of
the tissue.
The duration and intensity of the local acute inflammatory response
must be carefully regulated to control tissue damage and facilitate the
tissue-repair mechanisms that are necessary
for healing. TGF-B
(اختصار لinflammation للمنطقة بعدregulation repair يساعد فيfactor اهم- :حفظ
) Transforming growth factor-B
has been shown to play an important role in limiting the inflammatory
response. It also promotes accumulation and proliferation of fibroblasts
and the deposition of an extracellular matrix that is required for proper
tissue repair.
Clearly, the processes of leukocyte adhesion are of great importance in
the inflammatory response. A failure of proper leukocyte adhesion can
result in disease as exemplified by leukocyte-adhesion deficiency.
(LAD (genetic disease)
10
Systemic Acute-phase response
systemic Acute Phase Response The local inflammatory response is
accompanied by a systemic response known as the acute-phase
response (Figure 15-13). This response is marked by the induction of
1. fever,
2. increased synthesis of hormones such as ACTH and hydrocortisone,
3. increased production of white blood cells (leukocytosis),
4. and production of large number of acute-phase proteins in the
liver.
The increase in body temperature inhibits the growth of a number of
pathogens and appears to enhance the immune response to the
pathogen.
مهمC-reactive protein is a prototype acute-phase protein -تنتج من الكبدwhose serum level increases 1000-fold during an acute-phase
C –reactive protein (CRP): - it is found in munute (very little) amount in
normal condition.
non-specific لكنهinflammation لذلك هو اول فحص يطلب للتأكد من وجود
11
Lecture 18
Function of CRP
response. It is composed of five identical polypeptides held together by
noncovalent interactions. C-reactive protein binds to a wide variety of
microorganisms and activates complement, resulting in deposition of the
opsonin C3b on the surface of microorganisms. Phagocytic cells, which
express C3b receptors, can then readily phagocytose the C3b-coated
microorganisms.
Many systemic acute-phase effects are due to the combined action of IL-1,
TNF-a and IL-6 (see Figure 15-13).
figure 15-13 Overview of the organs and me diators involved in a systemic
acute-phase response. IL-1, IL-6, and TNF-a, which are produced by
activated macrophages at the site of inflammation, are particularly important
in mediating acute-phase effects. LIF = leukemia inhibitory factor; OSM =
oncostatin M.
12
Chronic Inflammatory Response
Chronic inflammation develops because of the 1) persistence of an antigen.
Some microorganisms, for example, have cell-wall components that enable
them to resist phagocy- tosis. Such organisms often induce a chronic
inflamma- tory response, resulting in significant tissue damage. Chronic
inflammation also occurs in a number of 2) autoimmune diseases in which
self-antigens continually activate T cells. Finally, chronic inflammation also
con tributes to the tissue damage and wasting associated with many types of
3) cancer.
The accumulation and activation of macrophages is the hallmark of chronic
inflammation. Cytokines released by the chronically activated macrophages
also stimulate fibroblast proliferation and collagen production. A type of scar
tissue develops at sites of chronic inflammation by a process called fibrosis.
Although fibrosis is a wound-healing reaction, it can interfere with normal
tissue function. Chronic inflammation often leads to formation of
granuloma.>>> This is a tumor-like mass consisting of a central area of
activated macrophages surrounded by activated lymphocytes. / The center of
the granuloma often contains multinucleated giant cells formed by the fusion
of activated macrophages. These giant cells typically are surrounded by large
modified macrophages that resemble epithelial cells and therefore are called
epithelioid cells.
13
Inflammation
inflammationسوف نتحدث عن موضوع جديد وهو
) للتحدث عن الموضوعch.3 in book( ثم سنرجع الى هناsheet لدراسة الموضوع سنذهب الى
CH.3: Mechanisms of innate immunity page (46)
– Inflammation - a response to tissue damage.
Stopping bleeding
Acute inflammation
Killing of pathogens, neutralizing toxins, limiting pathogen spread
Phagocytosis of debris, pathogens, and dead cells
Proliferation and mobilization of fibroblasts I or other tissue cells. To
contain an infection and/or repair damage
Removal or dissolution of blood clots and remodeling of the
components of the extracellular matrix
Regeneration of cells of the tissue and re-establishing normal
Structure and function
– Inflammation brings leukocytes to sites of infection or tissue damage
Q: what three principal changes occur in the tissue during an acute
inflammatory response?
A: An increased blood supply to the affected area (vasodilation),
increase in capillary permeability allowing larger an serum molecules to
enter the tissue and an increase in leukocyte migration into the tissues
(Extravasation)
Fig 3.2(edition9)6.2(edition 8) (The phased arrival of different
populations of leukocytes into a site of infection).
14
Y-axis والخاليا علىX-axis الحظ الوقت على
neutrophils الحظ أن أول خلية تصل وبكميات كبيرة (الحظ الشكل الكبير) هي
لكنmacrophage خالل هذه األثناء تصل,3 days هي التي تبدأ بالعمل وتستمر ل
,بكميات أقل
. ممكن يساعدوا بالعمليةB, CTL, TH
–
–
–
–
From book, page (47-51)
Leukocytes migrate across the endothelium of microvessels
Although the patterns of leukocyte migration are complex, the basic
mechanism appears be universal. The initial interactions are set out in a
three-step model:
3.7 شرح صورة3 step ال
– Step-1 leukocytes are slowed as they pass through a venule and roll
( )تتدحرجon the surface of the endothelium before being halted ( (تتوقفthis is mediated primarily by adhesion molecules Called selectins (
تبطئleukocyte) interacting with carbohydrates on glycoproteins.
– Step-2 the Slowed leukocyte now have the opportunity to respond to
signaling molecules held at the endothelial surface-particularly
important is the large group of cytokines called Chemokines, which
activate particular populations of leukocytes expressing the appropriate
chemokine receptor.
– Step-3 activation up regulates the affinity of the leukocytes integrins,
which new engage the cellular adhesion molecules on the endothelium
to cause firm adhesion and initiate a program of migration.
affinity وهذا يؤدي إلى زيادة, leukocytes لactivation يحدثchemokines (بسبب
adhesion molecules معintegrin's فترتبط, WBCs الموجودة علىintegrins ل
squeezing حتى تبدأ عمليةfirm ويكون هذا االرتباط, endothelial walls الموجودة على
) واالنتقال
15
Fig 3.3 edition 9(6.3 edition 8) (TNE a is a cytokine with many functions)
نحن نتحدث عن دورها في, التي تبين الوظائف المختلفة لهاTNFa الحظ األسهم الخارجة من
. بإمكانك االطالع على وظائفها األخرى, inflammation
Leukocyte traffic into tissues is determined by: 1. adhesion molecules
and 2. signaling molecules.
16
Fig 3.4, (6.4 edition 8) (intracellular signaling pathways induced by
TNF a)
سنتحدث عن خطوات, )TNFa( على الخليةreceptor حتى يقوم بعمله يجب أن يرتبط بTNFa
. cytokines بشكل مختصر الحقا ً عندما نتحدث عنactivation
17
Fig 3.5 (leukocytes adhering to the wall of a venule by EM) مرور
, Fig 3.6 (Leukocyte migration across endothelium) مرور
18
Fig 37 (Three- step model of leukocyte adhesion)
تحدثنا
عنها
قبل
,قليل
في
هذه العملية, وتتدحرجendothelial علىselectins الخطوة األولى الخلية تتباطأ عن طريق وجود
, tethering تسمى
،endothelium الموجودة علىchemokines لtethered علىreceptors في الخطوة الثانية يوجد
وترتبط بشكل قوي جدا ً علىWBCs الموجودة علىactivation of integrin's في الخطوة الثالثة يحدث
.endothelium الموجودة علىadhesion molecules
Selection bind to carbohydrates to slow the circulating
leukocytes. )(الخطوة األولى
19
Fig 3.8 (6.8) (Lymphocyte migration by EM) مرور
3.9 (Selection) مرور
20
Chemokines and other chemotactic molecules trigger the
tethered leukocytes.
)(الخطوة الثانية
, Q: what advantage is there in having different types of inflammation
occurring in different tissues?
A: what constitutes an appropriate immune response depends on the
pathogen, the amount of damage it is causing in a particular tissue, and the
capacity of that tissue to repair and regenerate.
Fig 3.10(6.10) (Chemokines) مرور
21
Fig 3.11 (Some chemokines receptors and their principal ligands) مرور
22
Integrin's on leukocytes bind to CAMs on the endothelium, ( الخطوة
)الثالثة
[CAMs: cell adhesion molecules]
Fig 3.12(6.12) (The affinity of integrins is controlled by inside-out
signaling) مرور
23
Fig 3.14(6.14) (Endothelial cell
adhesion molecules) مرور
Fig 3.15(6.15) (Chemokines and
cell migration into lymphoid
tissue) مرور
24
From book: (page 55,56)
) الخارجيةsheet (أخذناهم في
Mediators of inflammation:
The four major plasma enzyme systems that have an important role in
hemostasis and Control of inflammation are the,
Clotting system
Fibrin lytic (plasmin) system
Kinin System
Complement System
Fig3.16(6.16) (activation of the Kinin System) مرور
25
Page 119, Fig 6.17 (The plasmin system) ( مرورsheet ) أخذنا شرحهم في
26
Fig 6.18( table 3.1) (Inflammatory mediators)
أخذناهم بشكلsheet نحن في, table ووضعهم فيInflammatory mediators هنا جمع بعض
. لكم حرية من أي مكان تدرسوهم, مفصل
Q: what determines whether an immune response is acute of chronic?
A: Ultimately the outcome of an acute inflammatory response is related
to the fate of the antigen, If the initiating antigen or pathogen persists,
then leukocyte accumulation continues and a chronic inflammatory
reaction develops. If the antigen is cleared, then no farther leukocyte
activation antigen occurs and the inflammation resolves.
27
From book (page 57, 58, 59) ً هذا الموضوع مهم جدا ً جدا
Pathogen-associated molecular patterns:
Before the evolutionary development of B cells and T cells, organisms still
needed to recognize and react against microbial pathogens.
pathogens تستطيع مقاومةorganisms ومع ذلك كانت، متطورةB and T cell قبل لم تكن
Hence, a variety of soluble molecules and cell surface receptors
developed which were capable of recognizing distinctive molecule
structures a pathogen. Such Structures (pathogen )الموجودة علىare called
pathogen - associated molecular patterns (PAMPs) and the proteins which
recognize them are pattern recognition receptors (PRRS).
)pathogens الموجودة علىPAMPs موجود عنا بأجسامنا لتمييزPRRs(
Typical examples of PAMPs are 1) Carbohydrates, 2) lipoproteins and 3)
lipopolysaccharide Components of bacterial and fungal cell walls while
Some of PRRs recognize the distinctive 4) nucleic acids (e.g. dsRNA)
formed during viral replication.
There are three main types of PRR:
Secreted molecules present in serum and body fluids;
Receptors, present on the cell surface and on endocytic vesicles; and
Intra-cytoplasmic recognition molecules. ()داخل السيتوبالزم
The intra-cytoplasmic recognition molecules are particularly important for
macrophage mediated recognition of internalized pathogens.
وهذا مهم أكثر شيء في خلية، شيء يميزهcytoplasm ويوجد في، أدخلpathogen (بمعنى أن
. ) phagocytosis ألنها رئيسية في عمليةmacrophage
Some of the secreted molecules are acute phase protein ex: MBL, CRP …
(I.e. they are present in the blood and their levels increase during infection).
Indeed, the first of these molecules the be recognized was C-reactive protein
(CRP), which can increase by more than 1000-fold in serum, during infection
or inflammation. this protein has been used as a clinical marker of
inflammation for more than 70 years.
PRRS allow phagocytes to recognize pathogens
The binding of the pathogen to the phagocyte can be direct or indirect
(opsonization).
28
Direct recognition involves the surface receptors on the phagocytes
directly recognizing surface molecules on the pathogen
(They are related to each other without an intermediary between them)
indirect recognition involves the deposition of serum derived
molecules on to the pathogen surface and their subsequent binding
to receptors on the phagocytes {i.e the process of opsonization}
notes: opsonization:( coating with Ab/ complement)
•Phagocytes have receptors that recognize pathogens directly —>
directly mean (Without needing an intermediary EX: complement /Ab) so:
Even in the absence of opsonic, phagocytes have a number of receptors
that allow them to recognize PAMPs.
these include:
(PPRsأنواع المستقبالت التي هي احد انوع1-3)
1. scavenger receptors - preceptor types which is one of the types
PRPs2. carbohydrate receptors (ex: mannose receptors)
3. Toll-like receptors (TLRs)
the scavenger receptors and carbohydrate receptors are primarily
expressed on mono nuclear phagocytes
pag.58 fig.3.19
29
In this picture, observed indirect by opsnization in the right part of picture.
The left part is: Direct recognition of the existence of 3 types of receptors on
phagocytic cell. These receptors are directly linked to bacteria. These are
carbohydrate receptors (EX: lectin receptor), TLR, scavenger receptor
Toll -like Receptor active phagocytes and inflammatory reactions
The family of TLRs include 10 different receptors in human, many of
which are capable of recognizing different component
page59 table3.2
(toll-Like receptors)—> ليس للحفظ
•
note :(We note that different TLRs are linked to different pathogens so
that it can facilitate its operation by phagocytosis without the need for
an intermediary [Ab/complement/...])
30
We have a very important question
Q: why do bacteria not just mature their MAMPs So that they cannot be
recognized by innate Immune system-After all protein and teachings of
pathogens often mutate? -Why does bacteria not work mutalion for MAMPal,
and therefore the body cannot distinguish it?
- MAMPs= microbes-associated molecules patterns
This is a difficult question because it requires deep insight in to evolutionary
history of microbes, But money of the MEMPs are so fundamental to the
structure of the bacteria or Fungi cell wells that it is difficult to see how they
could be attached without destroying the integrity of the microbes
(MAMPs for Microbe, like the main mayor of the building, cannot work in it. If
the mutations occur, Microbe is destroyed, another type -except MAMPs- do
mutations (- be essential objects, not sub-)
Cytokines
characteristics of cytokines:
1) Cytokines are protein hormone like molecules composed of:
a) Monokines, which are cytokines produced by mononuclear phagocytes.
b) Lymphokines, which are cytokines produced by T-lymphocytes.
c) Colony stimulating factors (CSFs), which are cytokines produced by the
lymphocytes & mononuclear phagocytes to stimulate the production of the
different blood cells in the bone marrow.
2) Cytokines can have any of the following activities:
a) Autocrine action: act on the cell type that produce the cytokine.
b) Paracrine action: act on cells in the local area of secretion.
c) Endocrine action: act on cells distant from the site of production.
3) Cytokine production is a short-lived event, they are not preformed
molecules (NOT synthesized & stored before) & their synthesis is initiated
by new gene transcription.
4) One type of cytokine can regulate the production of other cytokines by
inhibiting OR enhancing their expression.
5) Multiple cytokines acting on a cell type can have inhibitory or synergistic
effect (interaction to produce more effect).
6) Many individual cytokines are produced by multiple diverse cell types.
31
7) Cytokines initiate their action on the target cell by binding to cellular
receptor specific for the cytokine, it activates intracellular signaling
pathway resulting in the production of active transcription factors, which
migrate to the nucleus. Examples o
Examples of cytokines according to their principle action:
1. Mediators of natural immunity e.g. IL-8 as chemotactic factor, TNF
(which activates mononuclear phagocytes & the neutrophils) & type 1
interferon (which inhibits viral replication within few hours).
2. Cytokines that regulate the lymphocytes (T & B-cells, adaptive
immunity), e.g. IL-2 (growth factor for T-cells, which increases the lytic
capability of NK cells) & IL-4 (B-cell) for isotype switching.
3. Mediators of inflammation, e.g. IL-1, IL-6 & TNF. 4) Stimulators of
hematopoiesis, e.g. IL-3 that acts on immature progenitor cells in the
bone marrow into all types of mature hematopoietic cells, GM-CSF
(granulocytes monocytes colony stimulating factor) & M-CSF
(monocyte colony stimulating factor).
**NOTE: cytokines have many functions that are critical (vital & important)
to immunity, but excessive production or action (cytokines storm) can lead
to tissue damage & even death.
32
Notice the
cytokines;
IL-3, GMCSF & MCSF. In
brief.
pag.48 fig.3.3
33
pag.49 fig..3.4
34
35
Lecture 19
Chpter6: major histocompatibility complex (MHC) (96)
In 1940s, Experiments for grafts between syngeneic animals (genetically identical) & allogenic
animals (NOT genetically identical, but the same species) established that there is genetic basis
for recognizing the graft as foreign.
That genes responsible for causing the grafted tissue to be accepted or NOT, were called histocompatible genes, although several different genes contribute to the rejection, single genetic
region is responsible for most of the rejection phenomena, so it was called major
histocompatibility complex. Thus, individuals who express the same MHC accept the grafted
tissue from one another, while individuals who differs in their MHC vigorously reject the grafted
tissue
In the late 1970s, it was recognized that T-lymphocytes do NOT recognize the antigen in its native
form; the antigen has to be processed & presented by APC in conjugation with MHC.
MHC genes also called Immune response genes.
MHC: proteins on the surface of nucleated cells that are coded for by multiple genes in a region on
chromosome 6, it is also called in human HLA (human leukocyte antigen) .
Clinical uses for MHC typing (NOT easy & NOT cheap)
→ Organ transplantation, we have to do MHC typing for the donor & the recipient & then
matching them (No way to be 100% identical, so we find the most compatible), because of
the polymorphism (very important term, it can express different alleles or forms of the
proteins in different individuals) of the MHC proteins, transplantation of organs between
individuals may be rejected. Also using of immune-suppressant drugs has increased the
success of the graft survival (must be tittered carefully, because it might result in cancers &
other infectious diseases).
→ Paternity testing: (used in Courts), typing for the mother, child & usually alleged father
are tested (NOT 100% accurate).
MHC disease association
In patients with certain diseases (some autoimmune diseases, some neurologic disorders, some
viral diseases & some allergies) particular HLA alleles are found more often than in healthy
individuals, e.g.
1) narcolepsy: brief attack of deep sleep, 100% of these patients have HLA-DR2, were as
only 22% of healthy individuals have this allele.
2) Ankylosing spondylitis: inflammation of the vertebrae, spinal deformities &
destruction of the cartilage, 90% of the patients have HLA-B27, were as 9% of healthy
individuals have this allele.
MHC haplotype
it is the total set of the MHC genes, the D region genes coding for class II MHC (DP, DQ & DR),
whereas A, B, C region genes coding for class I MHC. MHC genes are the most polymorphic
genes known in human.
MHC molecules
Recognition by the αβTCR requires antigen to be bound to an MHC molecule.
in humans the MHC is known as the HLA
The proteins responsible for presenting antigens to T cells, MHC class I and class II proteins, were
originally discovered as histocompatibility (transplantation) antigens. Histocompatibility refers to
the ability to accept tissue grafts from an unrelated donor. The major histocompatibility complex
locus (MHC) comprises over 100 separate genes and was discovered when it was recognized that
both donor and recipient had to possess the same MHC haplotype to avoid graft rejection. The
principal moieties that determine rejection were identified as MHC class I and class II molecules
Fig 6.1
We need to Know the human genes NOT the mice's one.
Notice Class I (D region
DP, DQ & DR) on the left.
Notice Class II (A, B, C –the three-principle ones- region) on the right.
Fig 6.5 (edition 8 5.5)
The real structure MHC class I.
Notice α1 & α2 chains & the cleft between them.
Notice the N terminals & the C terminals.
Notice β2m & α3 chains
From the book page
Genetic organization of the MHC
The number of gene loci for MHC class I and class II molecules varies between
species and between different haplotypes within each species, and many
polymorphic variants have been described at each of the loci.
The three principal human MHC class I loci are HLA-A, HLA-B, and HLA-CI.
HLA-E, HLA-F, HLA-G, and HLA-H are class I genes.
Fig 5.2
Notice the A, B, C regions with hundreds of genes between them.
Human MHC class II genes are located in the HLA-D region
DR, DQ, and DP
Fig 6.3
Notice the D region in the human genes, DP, DQ & DR.
MHC haplotype and disease susceptibility.
Genetic variations in MHC molecules affect:
>> The ability to make immune responses, including the level of
antibody production (T-cell recognizing the A.G presented on the MHC
activation of B-cell & differentiation into plasma cells
A.B
Production).
>> Resistance or susceptibility to infectious diseases.
>> Resistance or susceptibility to autoimmune diseases and allergies.
Knowing this, we can start to answer the question of why the MHC is so
polymorphic. The immune system must handle many different
pathogens.
Q. The haplotype HLA-B53 is associated with protection against
childhood malaria, a disease that is prevalent in equatorial regions. In
which country would you expect to find the highest frequency of the
HLA-B53 allele – China, Ghana, or South Africa?
A. The gene frequency is around 40% in Ghana and 1–2% in China and
South Africa, which are outside the equatorial regions affected by
malaria.
HLA molecules were originally defined serologically. For example, HLA-A2 refers to a group
of HLA molecules with various amino acid sequences, all of which can be recognized by anti-HLAA2 antibodies. Modern sequencing technologies mean that it is now faster and easier to define
HLA by the sequence of the genes that encode them. when they are defined by genotyping, we
call the HLA-A2 group of alleles HLA*A2. Defining alleles by genotype ng also yields information
about the precise amino acid sequence of the genes.
For example, HLA-A*0201 refers to a single molecule within the HLA-A2 serotype, which has a
specific sequence of amino acid residues.
From book 96
The Class I Region
In humans, the class I region contains six genes of these, HLA-A, HLA-B and HLA-C are known as
classical class I genes. They encode the heavy (or alpha) chains of molecular that present antigen
to CD8 T cells. The classical class I are highly polymorphic, with each having thousands of
possible variants or alleles. In contrast, there are only a few alleles of each of the non-classical
class I (or class Ib) genes, HLA-E, HLA-F genes and HLA-G.
The Class III Region
The genes in the class III region are very diverse. Some encode:
complement system molecules (C4, C2, factor B);
enzymes;
cytokines;
heat shock proteins;
molecules involved in antigen processing.
Class I MHC
found on almost all body cells with some exceptions including
erythrocytes & corneal epithelium.
it is composed of two non-covalently linked polypeptide chains (s-s):
α chain around 325 A.A long & β chain (β2 microglobulin, B2M) around
100 A.A.
class I: this structure is divided into 4 regions, the upper region is called
peptide binding region composed of α1 & α2 each is around 90 A.A
long, between them there is a region called cleft/groove, polymorphism
among class I occurs in the cleft to allow binding to broad range of
antigens.
the beneath region is called I.G like region (α3 + β2M) around 190 A.A
long, it is called so because the amino acid sequence is homologous to
the amino acid sequence in the constant domain in Immunoglobulin,
which is the binding site of the CD8.
The third region is called Trans plasma membrane region (does not
change) around 25 A.A long, it forms an α helix that passes through the
plasma membrane lipid bilayer.
The last region is called cytoplasmic region, found in the cytoplasm
around 4 A.A long so it is called cytoplasmic tail, it regulates the
interaction between class I & cytoskeletal elements.
Class II MHC
It is expressed on B-cells, macrophages, DC (professional APCs(
&other few cells.
It is composed of two non-covalently linked polypeptide chains (s-s), α
&β chains each around 205 A.A long (but the A.A sequence differs).
The α chain is divided into α1 & α2, & so do the β chain (into β1 & β2(
disulfide bond between them.
it is structurally divided into 4 regions, the last two regions are the
same in all receptors, the cytoplasmic region(tail), around 4 A.A, which
regulates the interaction between class II & cytoskeletal elements & the
trans plasma membrane region, around 25 A.A long, it forms an α helix
that passes through the plasma membrane lipid bilayer.
The first two regions have the same names as in class I MHC, but they
differ in their components.
The second region, I.G like region, it is called so because the amino acid
sequence is homologous to the amino acid sequence in the constant
domain in Immunoglobulin, it is composed of α2 & β2, around 180 A.A
long, it is the binding site for CD4.
The first region, peptide binding region, composed of α1 & β1, around
180A.A, between them there is a region called cleft/groove,
polymorphism among class II occurs in the cleft to allow binding to
broad range of antigens.
Fig 6.9
Comparison between the extracellular
domains of the MHC class I & MHC class
II.
Class I
α1 & α2
β2m & α3
Class II
α1 & β1
α2 & β2
From the book
Antigen presentation by MHC molecules
once the structures of the TCR and the MHC–peptide complex had been
established, the next question was to determine how they interacted.
Page 99, Fig 5.13(edition 8)
it shows the difference between the MHC & the TCR.
Fig 5.14(edition 8)
Lucture20
Chapter 7: Antigen Presentation
From the book 109
Antigen presenting cells
T cells only recognize antigen peptides bound to MHC encoded molecules.
Endogenous peptides, derived from intracellular sources such as replicating
viruses, are presented on MHC Class-I molecules to CD8+ T-cells, while
exogenous peptides, derived from extracellular sources such as microbes,
are presented on MHC class-II molecules to CD4+ T-cells.
Before peptides can associate with the MHC molecules, they are generated
by partial proteolysis from the original protein antigen
. Antigen processing: - refers to the degradation of antigen into peptide
fragments, which may become bound to MHC class I or class II molecules.
From the book page 109
Interactions with antigen-presenting cells direct T cell activation
The four main types of APC are:
1) DCs, which are most effective at presentation to naïve T cells.
2) Macrophages.
3) B cells.
4) Innate lymphoid cells
1
From the book, page 110 fig.7.2
2
Fig.7.3
Notice each type of cells & their way of antigen presentation on
class II MHC to the Th.
3
Chapter 7: antigen presentation
antigen processing: the conversion of the native protein
antigen into MHC associated peptide fragments.
Class II MHC antigen processing pathway:
Exogenous antigens: - are internalized by endocytosis & if in fluid form,
by pinocytosis into the endosomes, which contains the cellular
proteases, it begins the degradation into immunogenic peptides, then
the endosome fuse with a vesicle bearing class II MHC & the peptides
interact with the groove/cleft, this structure (the endosome &the
vesicle) will fuse the plasma membrane of the APC & the peptide will be
presented to T-helper lymphocyte.
Features of the process:
>> This process takes 1-3 hours to occur.
>> Class II MHC is synthesized in the rough endoplasmic reticulum &
transported through post Golgi vesicle.
>> Antigen processing takes place in acidic intracellular compartment,
because cellular proteases works optimally at acidic PH , so chemical
agents such as ammonium chloride are potent inhibitors for the Antigen
processing.
>> The cleft of class II accommodate peptides of 13-24 A.A long, while
class I accommodate peptides 5-15 A.A long (The protein antigen
escapes complete degradation when the endosome fuse with the
vesicle).
4
Antigen processing.
Antigen processing involves degrading the antigen into peptide
fragments.
Antigens are partially degraded before binding to MHC
molecules.
The processing of antigens to generate peptides that can bind to MHC
class II molecules occurs in intracellular organelles. Phagosomes
containing endocytosed proteins fuse with lysosomes where a number
of proteases are involved in breaking down the proteins to smaller
fragments.
The proteases include- Cathepsins B and D & an acidic thiol reductase.
Alkaline agents such as chloroquine or ammonium chloride diminish the
activity of proteases in the phagolysosomes and therefore interfere with
antigen processing.
MHC class II pathway
Class II molecules are loaded with exogenous peptides
5
From the book, page 150 fig.8.12(edition 8)
→ Notice that the antigen (in the middle) have been endocytosed to the
endosome, & then degraded into fragments called peptides by the proteases,
then on the left side there is a vesicle with MHC class II from the Golgi
apparatus, it will fuse with the endosome to make the endosome-vesicle
structure & the peptides are located at the cleft of MHC class II now.
→ Then this structure will fuse with the plasma membrane (at the right side), &
presented to the outside to the DC4+ cell.
نفس الرسمة في األعلى
Fig.7.10 (edition 9)
6
Class I MHC Antigen processing pathway:
Endogenous Antigens are processed by a separate & distinct path from
class II, the ONLY factor that determines whether class I or class II
pathway is whether the antigen is endogenous or exogenous.
>> Class I MHC & viral gene transcription leading to synthesis of class I in
the RER, while the viral proteins in the cytoplasm.
>> Uptake of viral proteins in the proteasomes for degradation.
>> Transport of the processed peptides into the RER, where it binds the
cleft of class I, then the peptide-Class I MHC are transported through the
Golgi for exocytic-vesicles.
>> Fusion of the vesicle with the plasma membrane of the APC &
presented to CTL.
MHC class I pathway
MHC class I-restricted T cells (CTLs) recognize endogenous antigens
synthesized within the target cell, whereas class II-restricted T cells (TH)
recognize exogenous antigen. Manipulation of the location of a protein
can determine whether it elicits an MHC class I - or class II-restricted
response.
Proteasomes are cytoplasmic organelles that degrade cytoplasmic
proteins
forms a barrel-like structure
Transporters move peptides to the ER
7
Fig 7.4
----------------------------------------------------------------------------------------From the book page 148, fig.8.8
Notice the proteasome in the middle where the antigen enters it &
being degraded into fragments, the endoplasmic reticulum at the upper
right starts synthesizing Class I MHC (α chain & B2m chain), and the
peptides are then transported to the cleft of the MHC MHC class I +
peptide.
8
Important notes
1) exogenous antigens are expressed on class II MHC to activate Th
lymphocytes that secretes cytokines, which promote antibody
production, which is most effective against extracellular microbes.
2) Endogenous antigens are expressed on class I MHC to activate CTL,
which is most effective against intracellular microbes (virally infected
cells, grafted cells & tumors), by perforin.
3) Self-MHC restriction: CD4+ cells recognize only antigens that are
presented on self-class II MHC, while CD8+ cells recognize only antigens
that are presented on self-class I MHC; because of this, the CMI (cellmediated immunity) cannot be transmitted between humans.
9
Froom bock 117
CD1 PATHWAY
CD1 molecules present lipids and glycolipids. CDI molecules encoded
outside the MHC on chromosome 1), are a family of non-polymorphic MHC
Class-I-like molecules that present lipids and glycolipids to subsets of T cells.
Humans have five CD1 genes whereas mice have two
. CD1 heavy chains are synthesized in the ER. Like class I molecules, they
assemble with B2 macroglobulin and are stabilized by the chaperones
calnexin, calreticulin and ERP75 during loading. ER-derived lipids are loaded
onto CDI molecules by a lipid transfer protein called MTP. The loaded CD1
molecules are then transported to the cell surface, except for CD1e, which is
transported to endosomes.
Fig 7.11
10
Chapter 6: T-cell receptors (102)
T-cell is a subset of lymphocytes, which is a small cell with a big nucleus
(which occupies most of the cell size) & cannot be distinguished from
the B-cell morphologically, so it is distinguished by its unique receptors.
It is the only MHC restricted cell (cannot recognize the antigen by its
normal form, it must be processed & presented by Antigen presenting
cells).
These studies on the T-cell receptors are one of the most important
advances in immunology in the last two decays. T-cell receptors: are
proteins that allow the cell to recognize & respond to the antigens, they
were studied using monoclonal antibodies, e.g. to study CD4+ cell we
use anti-CD4.
Examples:
1) TCR: found on both Th & CTL.
>> It is composed of heterodimer consisting of 2 polypeptide chains α &
β chains NON-covalently linked together (s-s), each chain is composed of
variable (102-119 A.A) & constant region (138-179 A.A)
α chain against β chain, N terminal & C terminal for each chain, the
disulfide bond in between.
the last region is the cytoplasmic region (tail), which regulates the
interaction with the cytoskeletal elements.
The third region, Trans plasma membrane region, 25 A.A long, it forms
an α helix that passes through the plasma membrane lipid bilayer. This
region is also called hinge region, which provides flexibility to the
receptor. The higher part is the variable region & just below it the
constant region.
11
>> Different antigen specifities (different TCR produced) generated by
altering the variable region of the TCR, the variable α chain is coded by V
& J regions -as the light chain in the antibody-, while the β chain is coded
by V, D & J region chains – as the heavy chain in the antibody-.
To calculate the diversity, we do as we’ve done in antibodies,
For the variable α region = V *J
For the variable β region = V *D*J
The whole diversity (rearrangement, recombination) of the TCR= α*β
>> Function of the TCR: Providing T-cells the ability to recognize the
peptide found on MHC complex.
2)CD3: It is also called CD3 complex (because it is composed of 6
polypeptide chains NON covalently bind to each other), it has No
polymorphism – do NOT bind with the Antigen- , TCR &CD3 are
physically associated in the rough endoplasmic reticulum where they are
synthesized & this association is required for their surface expression.
>> Function of CD3: transduction of activating signals.
12
From the book, page 89
T cell receptors
the immune system of higher vertebrates can be divided into two
components – humoral immunity and cell-mediated immunity.
>>Humoral immunity, of which antibodies are a key component,
provides protection via the extracellular fluids. Antibodies deal quite
effectively with extracellular pathogens:
1) Targeting them for phagocytosis or complement mediated lysis.
2) Neutralizing receptors on the surface of bacteria and viruses.
3) Inactivating circulating toxins.
>>Cell-mediated immunity: of which T cells are critical operatives. T
cells recognize antigen via specialized cell surface antigen receptors – T
cell receptors (TCRs) –, which are structurally and evolutionarily related
to antibodies.
13
TCRs recognize antigen via variable regions generated through V(D)J
recombination, much like immunoglobulins, but are much more
restricted in their antigen recognition capabilities.
TCRs recognize peptides displayed by MHC molecules
T cells generally recognize fragments of degraded proteins (peptides),
which must be bound to (‘presented by’) specialized antigen-presenting
molecules encoded by the major histocompatibility complex (MHC). In
humans the MHC was first identified as the human leukocyte antigen
(HLA).
Fig.6.3
both have the variable (antigen binding site) & constant regions.
However, they differ in that the variable region in the A.B is made up by
of the heavy chain& the light chain, but in the TCR is made of α & β
chains.
TCRs are similar to immunoglobulin molecules
14
Fig.6.17
Notice the TCR-Cd3 complex, theCD3 does NOT have a variable
region, so it is NOT polymorphic, it is a 6 polypeptides chain
molecule & they are associated with the TCR since it’s
synthesized.
15
From the book, page 104
TCR variable region gene diversity is generated by V(D)J
recombination
As with antibody genes, a highly diverse repertoire of TCR variable
region genes is generated during T cell differentiation by a process of
somatic gene rearrangement termed V (D)J recombination
Variable (V), joining(J), and sometimes diversity (D) gene segments are
joined together to form a completed variable region gene.
The mechanism of V(D)J recombination is the same in both T
cells and B cells
-----------------------------------------------------------------------------Q. Why is it that, unlike B cells, T cells have not evolved a class
switching mechanism?
A. Class switching is irrelevant because there is no secreted
form of the TCR and hence no interaction analogous to that of
immunoglobulin and FcR.
-----------------------------------------------------------------------------Recombination yields great diversity
4.4× 1013 different forms of TCR Vβ.
8.5× 1012 forms of TCR Vα. They estimate that if only 1% of the
sequences coded for able proteins this would still give2.9× 1022
receptors. 99% of these viable receptors were rejected due to auto
reactivity or other defects; recombination would still yield 2.9× 1020
enough potential diversity, given that the thymus produces fewer than
109 thymocytes over the lifetime of a mouse.
16
V(D)N recombination occurs first in the α and then in the B chain.
The B chain of the TCR, encoded by the TCRB locus, is the first to
undergo recombination. D-to-J recombination occurs first, followed by
V-to-DJ rearrangements. All gene segments between the V, D and J
segments in the newly formed complex are deleted.
V(D)J recombination relies on recombination activating genes (RAG) 1
and 2. V(D)J recombination requires a complex enzyme collectively
known as VDJ recombinase. The most important of these are:
recombination activating genes (RAG) 1 and 2;
terminal deoxynucleotidyl transferase (TdT)
THE T-CELL RECEPTOR COMPLEX
The CD3 complex associates with antigen-binding aß or γ δ
o heterodimers to form the complete TCR.
17
Lecture21
from the book page 105-106
The CD3 complex associates with the antigen binding αβ or γδ heterodimers to
form the complete TCR
The four members of the CD3 complex (γ, δ, ε, and ζ) are sometimes termed the
invariant chains of the TCR because they do not show variability in their amino acid
sequences.
BOOK: THE T-CELL RECEPTOR COMPLEX
The CD3 complex associates with antigen-binding aß or yo heterodimers to form
the complete TCR. One remarkable feature of the transmembrane portion of the
TCR is the presence of positively charged residues in both the a and B chains.
Unpaired charges would usually be unfavourable in a transmembrane region, but
these positive charges are neutralized by assembly of the complete TCR complex.
This complex contains additional polypeptide chains, collectively called the CD3
complex, which bear complementary negative charges.
The CD3 complex allows the antigen-binding domains of the TCR to form a
complete, functional receptor that is stably expressed at the cell surface and is
capable of transmitting a signal on binding to antigen.
The four chains of the CD3 complex (y, δ, ε and ζ) are sometimes termed the
invariant chains of the TCR because they do not show variability in their amino acid
sequences. (The y, and δ chains of the CD3 complex should not be confused with the
antigen-binding variable chains of the y, δ TCR that bear the same names.)
The CD3 y, δ and ɛ chains are the products of three closely linked genes and
similarities in their amino acid sequences suggest that they are evolutionarily
related. All three are members of the immunoglobulin superfamily, containing an
external domain followed by a transmembrane region containing negatively charged
amino acids and a highly conserved cytoplasmic tail of 40 or more amino acids.
The CD3 ζ gene is on a different chromosome from the CD3 y, δ, ε gene complex and
the ζ protein is structurally unrelated to the other CD3 components. The ζ chains
possess a small extracellular domain (nine amino acids), a transmembrane domain
carrying a negative charge and a large cytoplasmic tail.
The CD3 chains are assembled as heterodimers of y ε and δ ε subunits with a
homodimer of ζ chains, giving an overall TCR stoichiometry of (aß)2, Y, δ ε 2 and ζ 2,
suggesting that the TCR complex exists as a dimer. The negatively charged residues
in the transmembrane region of the CD3 chains interact with (and neutralize) the
positively charged amino acids in the aß polypeptides, leading to the formation of a
stable TCR complex (Fig. 6.17).
1
Fig. 6.17
The cytoplasmic portions of ζ and η chains contain ITAMs The cytoplasmic portions
of these subunits contain particular amino acid sequences called immune receptor
tyrosine-based activation motifs (ITAMs), and each chain contains three of these
motifs. The conserved tyrosine residues in the ITAM motifs are tar gets for
phosphorylation by specific protein kinases.
When the TCR is bound to its cognate antigen–MHC complex, the ITAM motifs
become phosphorylated within minutes in one of the first steps in T cell activation.
ITAMs:
1) are essential for T cell activation, and mutational substitution of the tyrosine's in
the motif prevents activation
2) Play critical roles in B cell activation, and are present in the B cell receptor chains,
Ig α and Ig β.
2
In addition to TCR & CD3, other proteins are needed for T-cell response to occur;
they were studied using monoclonal antibodies.
They increase the strength of binding between T-cells & APC, & they can determine
which MHC class is recognized by the T -cell, these proteins are called accessory
proteins. (3-7)
3) CD4: it consists of single polypeptide chain; it has four immunoglobulins like
domains >>> the amino acid sequence is homologous to the amino acid sequence in
the constant domain in Immunoglobulin.
It is present on around 65% on the mature T-lymphocyte (Th), it is also present but in
small quantities on macrophages. it is the receptor for HIV virus, so the Th cells are
the cells which gets infected with HIV & some macrophages.
Function of CD4:
it increases the strength of binding between CD4+ cell & APC with its specific affinity
for class II MHC by binding to immunoglobulin like region (α2 & β2).
3
4) CD8: it is composed of homodimer (only α) or heterodimer (α & β), it is present in
around 35% of mature CTL
Function of CD8: it increases the strength of binding between CD8+ cell & APC
with its specific affinity for class I MHC by binding to immunoglobulin like region (α3
& β2m).
CD4 & CD8 facilitate signal transduction.
5) CD2: it is present on more than 90% of mature T-lymphocyte, it is a single
polypeptide chain,
Function of CD2
it increase the strength of binding between Tcell & APC by binding leukocyte
function associated antigen-3 found on APC (LFA-3).
6) (LFA-1): found on more than 90% of mature T-lymphocyte it is also present on B-cells,
neutrophils & monocytes.
Function of LFA-1: it increase the strength of binding between the Tcell & APC by
binding intracellular adhesion molecules on APC (ICAM).
7) CD28: It is expressed initially; it binds another receptor called B-7 fond on APC to
induce activation. Once activation has peaked, and CD28 is replaced by CTL A-4 (Tcells) to stop activation.
4
Fig.7.1
Book (117-118)
Co-stimulation
Danger signals enhance T-cell activation. For appropriate immune responses to be
generated, T cells must respond to infection, but not to high levels of harmless
antigen that may fluctuate in the environment. Mucosal tissues in the gut are in
contact with high concentrations of harmless food antigens, while respiratory
mucosa contacts many airborne antigens such as pollen, but strong immune
responses against these antigens are undesirable.
APC activation is generally a response to infection or at least the presence of
substances, such as constituents of bacterial cell walls, that are characteristic of
infection. This explains the mechanism of action of adjuvants derived from bacterial
components, which can be used to enhance the immune response experimentally.
The concept of immune activation only in response to infection (or adjuvant as a
5
surrogate for infection), and not to other antigens, has been promoted as the danger
hypothesis.
In other words, foreign substances may be invisible to the immune system unless
accompanied by danger signals provided by pattern recognition receptors on APCS,
such as the Toll-like receptors (TLRS), which recognize microbial products. These
cause increased antigen presentation by upregulating MHC and adhesion molecules,
but also boost T-cell activation by increasing the expression of co-stimulatory
molecules.
Co-stimulation by CD80/86 binding to CD28 is essential for T-cell activation. T-cell
recognition of antigen presented on MHC molecules, although necessary, is not
sufficient to activate the I cell fully. A second signal, referred to as co-stimulation, is
of crucial importance for T-cell activation.
The most potent co-stimulatory molecules are B7s, which are homodimer members
of the immunoglobulin superfamily. They include CD80 (B7.1) and CD86 (B7.2).
CD28 is the main co-stimulatory ligand expressed on naive T cells. CD28 ligation:
prolongs and augments the production of IL-2 and other cytokines; and
prevents the induction of energy, a condition in which the T cell is not
activated and is subsequently unable to respond to antigen.
Fig 7.12
6
Note fig.7.12
Notice in the anergy state, the CD28 is not in the immunological synapse, so
the cell is unable to respond although the TCR is bound to the antigen.
Notice in the activation state, the CD28is bound to B7, the T-cell starts to
produce IL-2, which acts as autocrine to start division, differentiation effector
function.
Once the activation reaches the peak, CD28 is replaced by CTLA-4, which has
higher affinity with B7 than CD28, which will inactivate the T-cell.
Fig 7.13
Ligation of CTLA-4 and PD-1 inhibit T-cell activation. CTLA-4 is an alternative ligand
for CD80 and CD86, with a higher affinity than CD28. CTLA-4 is not a conventional
inhibitory receptor, since it lacks a bona fide immunotyrosine-based inhibition motif
(ITIM), which recruits phosphatases that inhibit signaling. It has been proposed that
it can recruit phosphatases indirectly or it may prevent T-cell activation by
preferentially binding CD80 and CD86, so that they are unavailable to bind CD28.
Following activation, T cells express higher levels of CTLA-4, which dampens their
ability to continue to respond to antigen presentation (Fig. 7.13).
Mice that lack CTLA-4 suffer from an aggressive lymphoproliferative disorder
because their T cells cannot b inactivated. A-4 PD-1 (programmed death-1, CD279) is
an inhibitory receptor expressed by T cells, which belongs to the same family as
CD28 and CTLA-4. Its expression is associated with an exhausted T-cell phenotype,
i.e. cells that are incapable of producing cytokines and undergoing further division.
PD-1 is ligated by PD-L1 and PD-L2 (CD273 and CD274) on APC and signals negatively
7
through its ITIM to inhibit the co- stimulatory signal from CD28. There is therefore a
balance in the co-stimulatory and inhibitory signals that a T cell receives, which
determines whether it remains in an active state.
From the book, page 119
T cell interaction with APCS The interactions between a T cell and an antigen
presenting cell develops over time, in three phases:
1. The initial encounter of T cells with APCs is by non-specific binding through
adhesion molecules, particularly ICAM-1 (CD54) on the APC and the integrin
LFA-1 (CD11/18), present on all immune cells. Transient binding permits the T
cell to interact with many APCs; T cells in vivo are highly active and a single T
cell may contact up to 5000 dendritic cells in one hour.
The initial phase of antigen presentation may last for several hours, but in the
absence of a specific interaction, the APC and T cell dissociate.
2. When the T cell encounters the appropriate MHC/peptide, a conformational
change in LFA-1 on the T cell, signaled via the TCR, results in tighter binding
to ICAM-1 and prolonged cell–cell contact. The joined cells can exist as a pair
for up to 12 hours, and this marks the second phase of interaction. At this
stage, an ‘immunological synapse’ forms and the T cell may be activated.
3.
In the third phase, the APC and T cell dissociate and the activated Tcell
undergoes several rounds of division and differentiation.
The immunological synapse is a highly ordered signaling structure
Fig.7.14
by florescence, the violet cell is the APC,
& just beneath it the T-cell, between the
two cells (the red region) is the
immunological synapse, the green pulleyed structure is the TCR.
8
TCRS, CD4, CD28 and CD2, which have relatively small extracellular domains, cluster
in the centre of the synapse (or central supramolecular activation cluster; CSMAC)
and the adhesion molecule LFA-1 forms a ring around the outside in the pSMAC
(peripheral SMAC). CD45, the common leukocyte antigen, is a phosphatase with a
large extracellular domain and is excluded from the synapse (Fig. 7.15). It is thought
that the size-based exclusion of this phosphatase allows the balance of enzymatic
activity within the synapse to be tipped in favour of phosphorylation, which triggers
T-cell signaling. T cells in which the molecules that usually segregate in the cSMAC
are artificially made to be the same size as CD45 cannot exclude CD45 from the
synapse and are unable to signal.
Fig 7.15
Notice that the TCR is located in the middle & surrounded by a group of adhesion
molecules. Notice that this cell is a CTL because we have CD8 & class I mMHC on the
APC.
9
T-cell activation(signalling)
the response of T-cells to peptide-MHC complex consist of series of cellular events
called T-cell activation which lead to proliferation by an autocrine growth pathway
in which the responding T-cell secrete its own growth promoting cytokines and their
receptors. The principle autocrine growth factor is IL-2
Clustering of receptors result in activation of tyrosine kinases (e.g. FYN, LCK) that
becomes activated by phosphatase domains.
FYN >>>activates phospholipase C which hydrolysis phosphatedil inositol 4, 5
bisphosphates into diacylglycerol + inositol 1, 4, 5 triphosphate
PIP2>>Ip3 + DAG
DAG activates Protein Kinase C, which phosphorylates many proteins some
of which are nuclear transcription factors.
IP3 releases Ca+2 from ER to activate Ca+2 dependent enzymes, e.g.
calcineurin, which removes phosphate from the transcription factor called
nuclear factor of activation of T-cells (NFAT). The transcription factors
translocate to the nucleus to activate genes including immediate early genes
for cell division, e.g. transcription of IL-2 gene & IL-2 receptor gene for
autocrine growth stimulation, & then mitosis begins.
)119+120(- :بعض الجمل من الكتاب
T-cell signalling requires phosphorylation of ITAMS. The 5 chains of the TCR ( بقصد
CD3), CD4 and CD28 all contain immunoreceptor tyrosine-based activation motifs
(ITAMS) in their cytoplasmic tails (see Chapter 6, the TCR complex). When they are
clustered together in the absence of the phosphatase CD45, the ITAMS initiate
signalling by recruiting tyrosine kinases. The most important of these are:
Lck, which is recruited by CD4 and phosphorylates ITAMS on the chains of the
TCR.
Fyn, which is recruited to the chains and phosphorylates phospholipase C
(PLCY). Phosphorylated PLCY cleaves a phospholipid component of the cell
membrane into two products, one of which promotes Ca2+ release from the
ER and influx from outside the cell. This is required for the activation of the
transcription factors NF-KB and NF-AT.
10
From the book, page120
Intracellular signaling pathways activate transcription factors an appropriate
stimulatory signal initiates a cascade of intracellular signals, leading to the activation
of transcription factor and expression of genes required for cell division. Two
widely-used immunosuppressive drugs, cyclosporin and tacrolimus, interfere with
the activation pathways.
Interleukin-2 drives T cell division T cell activation leads to the production of IL-2
and IL-2 receptors, so a T cell can act on itself and surrounding cells. In most CD4+
cells and some CD8+ T cells, there is a transient production of IL-2 for 1–2 days.
During this time the interaction of IL-2 with the high-affinity IL-2R results in T cell
division.
On resting T cells, the IL-2R is predominantly present as a low-affinity form consisting
of two polypeptide chains, a β chain (p75) that binds IL-2 and a commong γ chain
that signals to the cell. When the T cell is activated, it produces an α chain (CD25),
which contributes to IL-2 binding and, together with the β and γ c chains, forms the
high affinity receptor. IL-2 is internalized within 10–20 minutes and the β and γc
chains are degraded in lysosomes while the α chain is recycled to the cell surface.
Sustained signaling by IL-2 over several hours is needed to drive T-cell division.
Fig 7.16
11
The transient expression of the high-affinity IL-2R for about 1 week after stimulation
of the T cell, together with the induction of CTLA-4, helps limit T cell division. In the
absence of positive signals, the T cells will start to die by apoptosis. However, a few
remain alive to become memory cells. The life span of memory cells can be more
than 40 years in humans. In view of the importance of IL-2 in T-cell division, it was
surprising that the rare patients who lack CD25 (and CD25 knockout mice) develop
an immunoproliferative condition. These observations led to an awareness that IL-2
also has a regulatory function in T-cell development.
Other cytokines contribute to activation and division (IL-15/IL-4/IL-6/IL-1)
From book pag.121
Activated T cells signal back to APCS.
Antigen presentation is not a unidirectional process. Activated T cells:
release cytokines such macrophage colony stimulating factor (GM-CSF),
which activate APCS;
express CD40 ligand (CD40L; CD154), which binds to the co-stimulatory
molecule CD40 on the APC. In macrophages, this increases their production
of microbicidal substances, including reactive oxygen species and nitric oxide.
In B cells, CD40 ligation is important for class switching and differentiation to
plasma cells.
12
Lecture 22
From the book (178Ideally, an immune response is mounted quickly to clear away a pathogenic
challenge with the minimum of collateral damage and then the system is returned to
a resting state. The immune response is therefore subject to a variety of control
mechanisms. Additional mechanisms help regulate the levels of immunopathology
that are often a necessary side effect of pathogen elimination. An insufficient
immune response can result in an individual being overwhelmed by infection or the
failure to clear cancerous cells. An inappropriate or over-vigorous immune response
can lead to high levels of immunopathology or even autoimmunity. The balance
between these two is therefore critical.
At its most basic, an effective immune response is an out- come of the interplay
between antigen and a network of immunologically competent cells. This chapter
provides an overview of how immune responses are regulated by:
- antigen dose and route of administration;
-co-stimulation, cytokine milieu and chemokine gradients;
- regulatory T cells immune responses;
- feedback control through immunoglobulin;
- apoptosis following the resolution of the immune response. their suppression of
unwanted.
Regulation of the immune response: - (chapter12)
1. Regulation by antigen (characteristics of the immunogen)
الذيparticle يجب ان يكونimmune response نتعرض له يعمل،تحدثنا سابقا انه ليس كل شيء
- :نتعرض له خصائص معينة مثل
(مروا في مادة الفيرست) وهذا نوع منAccessibility, route of administration, complexity, size
regulation أنواع
2. Regulation by the antigen presenting cell (APC){professional and
nonprofessional}
3. T-CELL REGULATION OF THE IMMUNE RESPONSE Differentiation into CD4 TH
subsets is an important step in selecting effector functions. Naive CD4 T cells
are able differentiate into a variety of phenotypes, the best characterized and
understood being the THl, TH2 and TH17 phenotypes (Fig. 12.5), which are
associated with type 1, type 2 and type 3 (which are sometimes also called
type 17) cytokine responses, respectively. The differentiation fates of TH cells
are crucial to the generation of effective immunity. Factors that may influence
the differentiation of TH cells include:
1
the sites of antigen presentation;
co-stimulatory molecules involved in cognate cellular interactions;
peptide density and binding affinity-high MHC class II pep- tide density
favours TH1 or TH17, low densities favour TH2;
•APCS and the cytokines they produce; the cytokine profile and balance of
cytokines evoked by antigen; receptors expressed on the T cell;
activity of co-stimulatory molecules and hormones present in the local
environment;
Fig 12.5
-: من جدول مطلوب
TH1 SECRETES>> IL-2,
TFNɣ,TNFβ
TH2 SECRETES>>IL4,IL5,IL-10
TH17 SECRETES >> IL-17
2
وغيرهمTH1,TH2 TH17 ) الىthymes ممكن تصير (تتمايز فيnaïve CD4+ T cell الحظ الرسمة
Cytokine balance a major regulator of T-cell is differentiation.
TH cell subsets determine the type of immune response. It is clear that:
local patterns of cytokine and hormone expression help to select lymphocyte
effector mechanism; and
the polarized responses of CD4+ TH cells are based on their profile of
cytokine secretion. Type 1 cytokines including IFNY and IL-12, also promote:
macrophage activation;
antibody-dependent cell-mediated cytotoxicity; and
delayed-type hypersensitivity.
TH2 clones are typified by production of the type 2 cytokines IL-4, IL-5, IL-9, IL-10
and IL-13 (see Fig. 12.5). These cells vide optimal help for humoral immune
responses biased towards:
IgG1 and IgE isotype switching;
mucosal immunity;
stimulation of differentiation;
and IgA synthesis.
Fig 12.6
3
Fig 12.7
cytokines الحظ ان بعض
(األسهم الحمراء) على سبيلinhibition (االسم الخضراء) وبعضهاstimulation تعمل
تفرزmonocyte - : بينما مثال اخر،inhibition الذي يعملIL-10 تفرزTH2 المثال
. TH1 لactivation الذي يعملIL-12
4. immune regulation by selective cell migration
تسمىTH بشكل عام سنتحدث عن جزء منTH تحدثنا قبل قليل عن دور
regulatory T cell
Regulatory T cell exert important suppressive function: A naturally occurring population of CD4+ CD25+ regulatory T cell (Tregs) is generated
in the thymes.
)Tregs ) <<<< هذه هيTH (الموجود علىCD4+ باإلضافة الىCD25+ عليهاTH (يوجد جزء من
-
Tregs maintain peripheral tolerance and have important roles in the
prevention of autoimmune diseases such as type 1 diabetes.
)multiple autoimmune diseases الشخص سيحدث عنده،(أي خلل في هذه الخلية
Treg differentiation is induced by Foxp3.
4
Comparison of CD4+ CD25+ Tregs with naive and activated CD4+ T cells shows that
regulatory T cells selectively express Foxp3, (a member of the forkhead/winged helix
transcription factors essential for the development and function of CD4+ CD25+
Tregs.
Mutations in the Foxp3 gene cause immune dysregulation, polyendocrinopathy
enteropathy and X-linked syndrome (IPEX). Individuals with this disease have
increased autoimmune and inflammatory diseases.
Mechanisms of Treg suppression: - ( الوسائل التي تستخدمها حتى تعمل
suppression) نقاط4
سنذهب الىmechanisms قبل ان نتحدث عن
12.13 صورة
Fig 12.13
او ممكن ان تكونCD4+ CD25+ Foxp+ ممكن تتجه الن يكون عليهاnaïve CD4+ CD25- Foxp- الحظ
) CD4+ CD25- Foxp-( Foxp بالتالي ال يوجدCD25 لكن ليس عليهاCD4+
)واألخرى ال يوجدCD25 , Foxp لكن أحدهما عليهاTH (الخليتان
)(مهم جدا جدا جداmechanisms نتحدث عنfig.12.14 االن سنذهب الى
5
Fig.12.14 )(مهمه
Tregs may suppress by a variety of mechanisms: -
1) Via cell- to-cell contact (secreted or cell surface molecules such as CTLA-4
expression or membrane-bound TGFβ.
T cell activation ويؤدي الى حدوثInitially الذي يكون موجودCD28 (تحدثنا سابقا عن
B7 يرتبطان بCTLA4 , CD25 ,CTLA-4 الى القمه يأتي محلةactivation وعندما يصل
)APC علىReceptor (
(يسمىB7 {وعليهاAPC) Dendritic cell(DC) انظر للرسمة باألعلى على اقصى اليسار الحظ
وعلى يسارها خلية, ) Treg( ) Foxp3+ ( ) امام هذه الخلية يوجد خليةCD80/CD86 أيضا
,(Foxp3-( أخرى
عندما ترى الخليتين امامها سترتبطAPC , CD28 يوجدFoxp3- T Cell وعلىCTLA-4 يوجدTreg على
STOP activation <<< وبالتالي, CTLA-4 لhigher affinity ,B7 النCTLA-4 مع
2) Release of suppressor cytokines such as IL-10, IL-35, TGFβ
Transforming
growth factor β
6
) هذهIL-10, IL-35, TGFβ(suppressor cytokines تفرزFoxp3+(Treg) انظر للرسمة الحظ
.
Foxp3- لsuppression ستعملcytokines
3) IL-2 consumption (Tregs can express high levels of CD25, the IL-2 receptor).
انظر الرسمة الحظ في األعلىgrowth factor وهوT cell activation لعملcytokine هو اهمIL-2
Treg ،موجود في المنطقة ؟ الخليتان تتنافسان لالرتباط بهIL-2 , Foxp3- وفي األسفلFoxp3+(Treg)
T cell وبالتالي تتوقف عمليةIL-2 receptor بكميات أكبر الن عليها كميات كبيرة منIL-2 تستهلك
)T Cell activation مهم لIL-2 (النactivation
4) Cytolysis
killing/apoptosis التي تعملgranzymes تفرزFoxp3+ والحظ ان،انظر الرسمة اقصى اليمين
.للخلية
1 بصفحةregulation نعود لتكملة نقاط
4 regulation of immune response by immunoglobulins
by feedback inhibition which is property of IgG antibody
)انظر رسمة الفيرست عن هذا الموضوع (الرسم ضفناها هون
5. APOPTOSIS IN THE IMMUNE SYSTEM
Apoptosis is a cellular clearance mechanism through which homeostasis is
maintained. Unlike cell damage-induced death (i.e. necrosis), which can
trigger immune responses, apoptosis maintains intracellular structures within
the cell. Apoptotic cells undergo nuclear fragmentation and the condensation
of cytoplasm, plasma membrane. and organelles into apoptotic bodies.
7
Apoptotic cells are rapidly phagocytosed by macrophages, which prevents
the release of toxic cellular components into tissues, so avoiding immune
responses to the dead cells.
At the End of an Immune Response, Antigen-Specific cell die by apoptosis
Following resolution of an immune response, the majority of antigen-specific
cells die by apoptosis. This ensures that no unwanted effector cells remain
and also maintains a constant number of cells in the immune system.
Apoptosis is controlled by a number of factors in the cell and depends on
expression of the death trigger molecule CD95 (Fas). Deficiencies in the FAS/
FASL(L=ligand) pathway can give rise to lymphoproliferative disorders with
autoimmune manifestations.
<<<< الخلية تموت وتصبح تطلق محتوياتها للخارج بما انها اطلقت محتوياتها للخارجNecrosis
IR << ممكن تحفز
<<<<ال تطلق محتوياتها للخارج بل تحتفظ بهاApoptosis
6. METABOLIC REGULATION OF THE IMMUNE RESPONSE ( جديده مش موجودة بنسخه
) كتاب قبل
Immune responses are bio-energetically expensive and it is becoming increasingly
apparent that metabolic pathways regulate T-cell differentiation and effector
functions in vivo. This is not surprising given that during T-cell and B-cell responses,
the immune system must meet the energetic demands to synthesize
macromolecules, such as proteins and DNA, whilst also producing ATP. It is notable
that many lymph nodes are located within adipose tissue, where they have a ready
access to precursors released by activation of the adipocytes by cytokines. Three
major pathways regulate energy demands: glycolysis, the tricarboxylic acid (TCA)
cycle and mitochondrial oxidative phosphorylation (OXPHOS).
(CHAPTER 2)(p.20)
Adipocytes produce inflammatory cytokines. Over the last 10 years it has
become clear that adipocytes are players in immune reactions. Although
adipose tissue was thought to be solely() حصرياinvolved in energy storage and
release, the metabolic and immunological functions of preadipocytes and
adipocytes have emerged. They are potent producers of pro-inflammatory
cytokines (e.g. IL-6 and TNFa) and chemokines, regulating
monocyte/macrophage function and they produce other molecules associated
with the innate immune system, such as the C1qrelated superfamily. Finally,
preadipocytes and adipocytes express a broad spectrum of functional Toll-like
receptors and can convert into macrophage-like cells and MHC molecules.
They are thought to play an important role in the inflammation that occurs in
type 2 diabetes.
8
T-CELL ACTIVATION INVOLVES A SWITCH FROM OXPHOS TO GLYCOLYSIS
Naive T-cell metabolic requirements are maintained principally through OXPHOS of
glucose and fatty acids. IL-7 signaling in combination with TCR signalling maintains
expression of the glucose transporter GLUTI on naive T cells, allowing glucose to
enter the cell (Fig. 12.20)
However, T-cell activation induces considerable metabolic reprogramming. TCR
signalling acts via ERK signalling pathways to promote uptake and breakdown of
glutamine, which is an essential component to replenish TCA cycle intermediates for
macromolecule synthesis. In addition, CD28 co-stimulation activates the PI3K/AKT
pathway to induce further expression of GLUT1 and switch metabolism from
OXPHOS to glycolysis. Interestingly, recent work has shown that activation of
glycolysis may play a pivotal role in controlling expression of the cytokines IL-2 and
IFNY in T cells as a result of translational control.
Fif.12.20
9
7. Immune regulation selective cell migration:
The spatial and temporal production of chemokines () شرطي المرور في المناعةby
different cell types is an important mechanism of immune regulation.
8. NEUROENDOCRINE REGULATION OF IMMUNE RESPONSES It is now widely
accepted that there is extensive cross-talk between the neuroendocrine and
immune systems. Both systems share similar ligands and receptors that
permit intra- and intersystem communication. These networks of
communication are deemed essential for normal physiological function and
good health. For instance, they play important roles in modulating the body's
response to stress, injury, disease and infection. The interconnections of the
nervous, endocrine and immune systems are depicted in (Fig. 12.21).
Fig. 12.21
10
growth افرازات الغدد (مثالhypothalamus الحظ الغدد الموجودة في الوسط في األعلى يوجد
منinsulin , thymus منT cell ,thyroid منthyroxine وanterior pituitary منhormone
) كلها تصبadrenal منcorticosteroids , gonads منsex hormone , islets of pancreas
sympathetic( الحظ السهم األزرق,لهمreceptor سيكون هناك, lymphoid tissue (على اليمين ) في
حتى محافظ علىnervous , endocrine, immune بينinteraction ) وبالتالي هذا يدل على وجود
. normal physiological function
Lymphocyte express receptor for many hormone, neurotransmitters, and
neuropeptides.
Corticosteroids are immunosuppressive. Corticosteroids, endorphins and
enkephalins, all of which may be released during stress Such hormones can have
strong effects on lymphocyte proliferation and can bring about the reactivation of
latent viral infections.
)herpes (من أشهرهم عائلة
Corticosteroids:
inhibit TH1 cytokine production while sparing TH2 responses.
lymphocyte تثبطcorticosteroids بالتاليT cell activation المهمين فيIFN ɣ,IL-2 تعطيTH1(
) TH2 بدون التأثير علىactivation
induce the production of TGFB, which in turn may inhibit the immune response.
Suppressor cytokines
-----------------------------------------------------------------------------------------------Q: In whet other ways doesIL-1 mediate interactions between the immune and
nervous system?
A: Causes an increase in body temperature (hypothalamus )على. Suppresses apatite,
and enhance the duration of slow-wave sleep
-------------------------------------------------------------------------------------------------Sex hormones affect immune cell function. Gender-based differences in immune
responses also occur. Immune cells have been shown to express receptors for
estrogens and androgens and it is likely that circulating levels of these hormones can
affect their function. It is noted that, during reproductive years, females
demonstrate more pronounced humoral and cellular immunity than males.
)تكون مناعتها اقوى من مناعة الرجلfemales لreproductive years (خالل
11
Some autoimmune diseases also show a gender bias. The systemic autoimmune
disease systemic lupus erythematosus (SLE) is 10 times more common in females
than males.
9. Genetic influences on the immune response
Familial patterns of susceptibility to infectious agents Suggest that resistance
or Susceptibility might be an Inherited characteristic. Such pattems af
resistance and susceptibility also occur in autoimmune diseases. Many genes
are involved in governing susceptibly of resistance to disease and the disease
is said to be under polygenic Control. ألنهmany genes
Considerable advances have been made in mapping and identifying the genes
governing the response to Some diseases as a result of: أسباب القدرة على عمل
خريطة جينيه
o the development of techniques Such.as microsatellite mapping.
o increased availability of DNA Samples
o sequencing of the human genome
MHC haplotypes influence the ability to respond to an antigen.
IR مثال على جينات لها تأثير على
Certain HLA(MHC) haplotypes confer protection from infection
لsusceptibility وبعضها يزيدinfection ضدprotection تعطييHLA haplotypes بعض
) infection
Many non- MHC genes also modulate immune responses
EX: - genes of complement, genes of cytokines genes of chemokines.
Polymorphisms in cytokine and Chemokine gene affect susceptibility to
infections
12
Page.197 11.21Genetic defects associated with immune deficiency or
abnormalities (edition 8)
CH:18 primary immunodefiencies هذا الجول سيعطى بشكل أكبر في،سنذكر بعض األمثلة حاليا
.(genetic diseases)
(severe combined immunadefiency) SCID أحد األمثلة هو
defect لوجود أكثر من
ألنه الوضع جدا سيء للطفل يولد وبعد شهر يموت
IL-2R α هوdefective gene فعند الطفل يكون
Result: - failure of IL-2 signal in activation and development.
RAG1/2 هوdefective gene وقد يكون
Result: - failure in TCR and BCR gene recombination.
التي يتعرض لهاdifferent ags وبالتالي لت يستطيع الكلل ان يتجاوب مع
13
Chapter 13: - immune response in tissue (192)
Immune response is not the same between tissue.
مثال ويتقبلهbrain فيtissue damage فال يعقل ان يكونtissue damage, ينتج عنهIR )
) اخرorgan الجسم كأي
. skin،Gut and lung ,CNS فيIR → سنتحدث في هذا التشابتير عن
TISSUE-SPECIFIC IMMUNE RESPONSES
What determines whether an immune response should consist of, for example,
activated cytotoxic T lymphocytes (CTLS) or a particular class of antibodies?
(pathogen) Although immune responses are primarily tailored to the 1) pathogen,
there is also a strong 2) influence from the local tissue, where the immune
response occurs (Fig. 13.1).
؟؟...... CTL اوAb ما الذي يحدد هل يكون التجاوب المناعي نوع من أنواع
Ag نوعية.1
مكان التجاوب المناعي في الجسم.2
This chapter focuses on:
the features of immune responses that are unique to individual tissues;
the mechanisms by which the tissues influence local and systemic characteristics.
There are several reasons why a particular organ may need to modify local
immunity. For example, tissues such as liver or skin have substantial capacity for
regeneration and a CTL response to kill virally infected cells is advantageous. In
contrast, the capacity for regeneration of neurons in the central nervous system
(CNS) is very limited and infected cells are often resistant to killing by CTLS: the
infection is controlled but not eradicated.
Herpes simplex and Herpes zoster have exploited this ecological niche and can
remain latent in neurons for many years, sporadically reactivating to produce cold
sores or shingles, respectively.
These observations suggest that the immune responses in tissues are
modulated in order to be appropriate for that site and even within one tissue
there may be micro-environments that have their own physiology and
preferred immune reactivity.
14
-
Some tissues are immunologically privileged. There are certain sites in the
body where fully allogeneic tissue can be transplanted without risk of
rejection. These include the anterior chamber of the eye, the brain and
testes. Several factors may contribute to the immunological privilege of
these sites, some of which affect the initiation of the immune response and
some affect the effector phase.
: -immunological privilege
محمية من المناعة (ال يوج فيها مناعة) لكن مع الوقت ثبت ان هذا الكالم غير صحيح
-
The privileged sites were long thought to be location where adaptive immune
responses are so dangerous that the immune system is not allowed entry, is
destroyed upon arrival or is prevented from functioning. Recent evidence
suggests that the concept of privileged sites may have been a misconception
based on the limitations of the experimental systems. Once the experiments
were expanded to include a wider variety of assays, it became apparent that
privileged sites are not immunologically impaired. They are simply sites that
are able to promote certain kinds of beneficial classes of immune responses
while suppressing classes that can do irreparable local damage.
يمنع من الدخول يدمر عند الوصول بمنع من أداء الوظيفة-
-
Locally produced cytokines and chemokines influence tissue-specific
immune responses. A number of factors control the type of immune
response occurring in each tissue:
1. The vascular endothelium plays a major role in determining which
leukocytes will enter the tissue by secretion of distinct blends of chemokines
and expression of site-specific adhesion molecules.
2. Cells in the tissue can also exert their effects via cytokines/ chemokines and
by direct cell-cell interactions. In effect, cells T signal infection damage or
stress and of the tissue can modulate immune cell activation.
-:1 توضيح لنقطة
Endothelium controls which leukocytes enter a tissue. Migration of
leukocytes into different tissues of the body is dependent on the vascular
endothelium in each tissue. For many thought that the endothelium in
different tissues, it was essentially similar, with the possible exception of
tissues. However, it was also well known that inflammation in different
tissues had different characteristics, even when the such as the brain and
retina, which have barrier properties inducing agents were similar.
15
It is now clear that a major element controlling inflammation and the
immune response is the vascular endothelium in each tissue, which has its
own characteristics; different endothelia produce distinctive blends of
chemokines (Table 13.1) and have their own sets of adhesion molecules to
mediate leukocyte transmigration into the tissue.
In addition, the endothelium can transport chemokines produced by cells in
the tissue from the basal to the luminal surface by transcytosis or by surface
diffusion in tissues that lack barrier properties (Fig. 13.2).
The surface (glycocalyx) of vascular endothelium also varies considerably
between tissues and this affects which chemokines are retained on the
luminal surface to signal to circulating leukocytes.
Table13.3
16
Fig 13.2
normal الحظ ان في،على اليسارnormal endothelium على اليمين وbarrier endo-thulium الحظ
, barrier endo. فيtight junction الحظ,endothelium براحتها منchemokines تمرendothelium
endothelium of different tissues <<< يوجد اختالف فيchemokines فتمنع مرور
17
Lecture 23
)) اخر محاضرة بمادة السكند
From book (194-201)
MMUNE REACTIONS IN THE CNS
1)
2)
3)
4)
5)
6)
The CNS, including the brain, spinal cord and retina of the eye, is substantially
shielded from immune reactions. The peripheral nervous system is also
partially protected. The low levels of immune reactivity in the brain are
ascribed to a number of factors:
The blood brain barrier (endothelium plus astrocytes) prevents the movement
of over 99% of large serum proteins into the brain tissue (IgG, complement,
etc.); there are similar barriers in the eye (blood-retinal barrier).
Low levels of major histocompatibility complex (MHO). molecule expression
and co-stimulatory molecules result in inefficient antigen presentation. The
brain lacks (leukocytel dendritic cells and these molecules are normally at low
levels on microglia, the brain's resident mononuclear phagocyte population.
There are no conventional lymphatics in the CNS, although drainage can occur
to cervical lymph nodes through the cribriform plate. The meninges do have
lymphatics and immune reactions in meninges have different characteristics
from those within the brain.
Low levels of leukocyte traffic into the CNS compared with other tissues.
Neurons have direct immunosuppressive actions on glial cells. Astrocytes,
neurons and some glial cells produce immunosuppressive cytokines. ( ستشرح
)باالمام
Some neurons and glia are protected against CTLS because they express Fasligand (death trigger molecule ) يحفز موت الخليةand can induce apoptosis in the
CTL, using one of the mechanisms normally used by CTLS The blood-brain
barrier excludes most antibodies from the CNS.
.... شرح العامل الخامس من العوامل السابقة
Later work showed that the electrical activity of the neurons Is important: neurons
that are functioning normally can suppress their neighboring glial cells (and
downregulate their own MHC molecules), whereas damaged neurons lift the local
immunosuppression to allow an immune response to develop.
للخالياsuppression فلم تعد قادرة على عمل،damage حصل لهاneurons مثال بعض
وهكذا,damage فيحدث فيهاMHC لdownregulation المجاورة<<< لم تعد قادرة على عمل
لذلك يجب ان تكون,brain فيimmune response ويحدث,damage خلية بعد خلية يحدث
MHC لdownregulation لمجاورتها وتعملsuppression حتى تعملactive الخاليا
) ببطء فيهاdamage يحدثbrain (لذلك امراض.الخاصة بها
18
Immunosuppressive cytokines regulate immunity in the normal CNS. Observations
of immune reactions in the CNS have led to the view that TH1- type immune
responses with macrophage activation and IFNY/TNFa production or TH17- Type
responses are most damaging. By comparison, TH2-type immune responses are less
damaging and strains of animals that make strong antibody responses against CNS
antigens are often less susceptible to CNS pathology than strains that make weaker
antibody responses.
نحن بحاجة اليها حتى تعطي مناعة ضد,most damaging تكونcytokines منTH1 انتاجات
damage لكن وجودها بالمنطقة يعملdifferent Ags
.Less damaging لكنIR في الدماغ لننا بحاجة ان يكون هناكTH2 نتوقع ان يكون
such observations have led to the view that immune deviation (the switching of an
immune response from TH1 towards a TH2 type) can be protective in the CNS.
This view is supported by findings that cells of the normal CNS can produce cytokines
associated with TH2 responses. For example, astrocytes produce TGFB and microglia
produce IL-10 when cultured with T cells and both astrocytes and neurons secrete
prostaglandins.
Immunosuppressive cytokines
immune reactions in CNS damage Oligodendrocytes.
Oligodendrocytes: - are glial cells that produce the myelin sheaths, which act
as electrical insulation around nerve axons. When immune reactions occur in
the CNS, these cells appear to be particularly vulnerable (Fig. 13.7). For
example, multiple sclerosis is characterized by focal areas of myelin loss
called plaques, typically a few millimeters in diameter.
Nerve transmission through these demyelinated areas is seriously impaired,
which may cause disease symptoms such as weakness and loss of sensation.
But it is only in the la stages of the disease that the neurons themselves are
damaged. The reason that oligodendrocytes are vulnerable in multiple
sclerosis is less clear
19
Fig 13.5
20
Fig 13.7
MMUNE REACTIONS IN THE EYE The eye
is a complex organ and subject to immune privilege. Indeed, the retina and optic
nerve are extensions of the CNS with neurons, glia and a blood-retinal barrier, which
is analogous to the blood-brain barrier. Also, like the CNS, the eye lacks a
conventional lymphatic drainage system.
Allogeneic) (من شخص لشخص اخرcorneal grafting is usually successful as a result of
immune privilege, although rejection may occur: the eye has very limited selfregenerative capacity and can be completely destroyed by a cell-mediated immune
response with the concomitant local production of TNFa and IFNY.
The eye has therefore evolved several major mechanisms to suppress cell-mediated
responses actively.
First, the epithelial cells lining the anterior chamber and the cornea express Fas
ligand; corneal allografts that lack Fas ligand (in rats) are almost always rejected.
Second, the fluid of the anterior chamber contains cytokines such as TGFB and IL-10,
which deviate the immune response towards the less-damaging TH2 type and
promote development of regulatory T cells.
Third, the cells of the iris and ciliary body secrete immuno- modulatory cytokines,
including TGFB.
21
Fig 13.8
- :lung and gut سنتحدث االن عن المناعة في
IMMUNE RESPONSES IN THE GUT AND LUNG
In contrast to the CNS, the gut and lung are examples of tissues that are
continuously in contact with high levels of harmless commensal organisms and
innocuous antigens as well as potential pathogens. It is essential that the immune
system in the gut does not make strong immune responses against antigens in food
or harmless commensal bacteria. (Note that food antigens are primarily present in
the small intestine, whereas bacterial antigens predominate in the large intestine.)
Similarly, many airborne antigens (e.g. pollen) are harmless and a strong immune
response in the lung is inappropriate: it would be considered hypersensitivity.
gut and lung فيIgA نوع المناعة هنا هو
The gut immune system tolerates many antigens but reacts to pathogens.
There many examples where an individual encounters an antigen in food and
subsequently becomes tolerant to it. This phenomenon is called oral tolerance and it
is related to nasal tolerance where antigen delivered to nasal mucosa as an aerosol
inhibits subsequent immunization.
22
Oral tolerance illustrates two points:
→ Tolerance can extend systemically to non-mucosal sites.
→ it is transferable to naive individuals by CD4 (or occasionally CD8) T cells.
Chronic Inflammation in the Gut
The most common types of chronic inflammatory disease of the gut are
inflammatory bowel disease (IBD), including *Crohn's disease, which may affect the
entire intestinal tract, and* ulcerative colitis affecting the large intestine. Both of
these conditions are thought to occur because of an overreaction to intestinal
bacteria; both have a strong genetic component and pattern recognition receptors
(e.g. NOD2) are disease susceptibility loci. In* coeliac disease, the patient is sensitive
to gluten in the food and susceptibility is strongly related to HLA haplotype and to
loci for inflammatory cytokines and chemokines.
In all three conditions, ulcerative colitis, coeliac disease and Crohn's disease, there is
production of TNFA and IFNY, dysregulation of the normal mucosal immune
response and damage to tissue, including the epithelium responsible for absorbing
nutrient chronic inflammatory diseases all appear to be a result of a reduction of the
normal tolerogenic mechanisms in the gut, so that the immune response is tipped
towards inflammation.
These Immune Responses in the Lung
The lung is another example of a tissue that is in normal contact with external
antigens and pathogens, including bacteria from the upper airways.
Bronchioles and alveoli contain large numbers of pulmonary macrophages, while
the lung tissue also contains lymphocytes and respiratory (plasmacytoid) dendritic
cells (PDCS) (Fig. 13.11). The macrophages and pDCs are major sources of IFNa and
IFNB, which are particularly important in controlling the initial spread of viral
infections.
Early recruitment of leukocytes to the lung is mediated by chemokines secreted
by the macrophages, pDCs and the epithelial cells of the lung itself (pneumocystis).
Neutrophils and natural killer (NK) cells are the first cells to appear after infection,
while dendritic cells traffic to the local bronchus-associated lymphoid tissue
(BALT).
Migration of the dendritic cells normally occurs at a steady rate, but following
infection there is an increase in movement of pDCs to the lymphoid tissues and,
and increase their expression of MHC class II and costimulatory molecules
CD80/86 and CD40.
23
Fig 13.11
IMMUNE REACTIONS IN THE LIVER The liver
The liver has an open circulation and receives blood from both the hepatic artery
and the portal vein and therefore it is potentially in contact with any bacteria or
bacterial products that have invaded the gut.
It is estimated that 99% o1 LPS that enters the liver is removed during transit, thus
protecting other tissues. Kuepfer cells that line the liver sinusoids and constitute
more than 90% of all tissue macrophages are a major element of the liver immune
system.
IMMUNE REACTIONS IN THE SKIN The skin
is the largest organ of the body and in humans there may be up to 106 T
cells/cm, in the dermis, tissue macrophages and at least two major populations
of dendritic cells, the Langerhans cells in the epidermis and the plasmacytoid
dendritic call mostly in the dermis.
→ The skin is very powerful
24
CONCLUSIONS
The immune responses and inflammatory reactions that occur in each tissue are
distinctive and are directed by interactions between the endothelial chemokines and
adhesion molecules and the circulating leukocytes. The endothelium in each tissue
synthesizes distinct sets of chemokines, and expresses specific adhesion molecules,
which attracts distinct leukocyte subsets.
نهاية مادة السكند
""اللهم انفعنا بما علمتنا وعلمنا ما ينفعنا وزدنا علما
25
26