Banana Waste as Renewable Energy Source: Characterization Study
advertisement

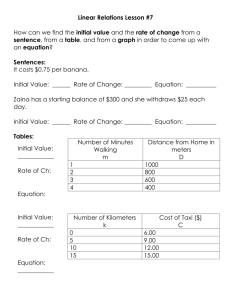
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/256483880 Characterization of Banana (Musa spp.) Plantation Wastes as a Potential Renewable Energy Source ARTICLE in AIP CONFERENCE PROCEEDINGS · JANUARY 2013 DOI: 10.1063/1.4803618 CITATION READS 1 164 3 AUTHORS, INCLUDING: Nurhayati Abdullah Rahmad Mohd Taib Universiti Sains Malaysia, George Town, Mala… UNISEL | Universiti Selangor 24 PUBLICATIONS 241 CITATIONS 6 PUBLICATIONS 1 CITATION SEE PROFILE SEE PROFILE Available from: Rahmad Mohd Taib Retrieved on: 07 November 2015 Characterization of banana (Musa spp.) plantation wastes as a potential renewable energy source Nurhayati Abdullah, Fauziah Sulaiman, and Rahmad Mohd Taib Citation: AIP Conf. Proc. 1528, 325 (2013); doi: 10.1063/1.4803618 View online: http://dx.doi.org/10.1063/1.4803618 View Table of Contents: http://proceedings.aip.org/dbt/dbt.jsp?KEY=APCPCS&Volume=1528&Issue=1 Published by the AIP Publishing LLC. Additional information on AIP Conf. Proc. Journal Homepage: http://proceedings.aip.org/ Journal Information: http://proceedings.aip.org/about/about_the_proceedings Top downloads: http://proceedings.aip.org/dbt/most_downloaded.jsp?KEY=APCPCS Information for Authors: http://proceedings.aip.org/authors/information_for_authors Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions Characterization of Banana (Musa spp.) Plantation Wastes as a Potential Renewable Energy Source Nurhayati Abdullah, Fauziah Sulaiman and Rahmad Mohd Taib School of Physics, Universiti Sains Malaysia, 11800 USM Pulau Pinang, Malaysia Abstract. Agricultural residue such as banana waste is one of the biomass categories that can be used for conversion to bio-char, bio-oil, and gases by using thermochemical process. The aim of this work is to characterize banana leaves and pseudo-stem through proximate analysis, elemental analysis, chemical analysis, thermo-gravimetric analysis, and heating calorific value. The ash contents of the banana leaves and pseudo-stem are 7.5 mf wt.% and 11.0 mf wt.%, while the carbon content of banana leaf and pseudo-stem are 42.4 mf wt.% and 37.9 mf wt.%, respectively. The measured heating value of banana leaf and pseudo-stem are 17.7MJ/kg and 15.5MJ/kg, respectively. By chemical analysis, the lignin, cellulose, and hemicellulose contents in the samples will also be presented. The potential of the banana wastes to be a feedstock for thermochemical process in comparison with other biomass will be discussed in this paper. Keywords. Renewable Energy; Biomass; Banana Waste; Thermochemical Characteristics. PACS NO. 88.20.hj INTRODUCTION Banana is the second most widely cultivated fruit, covering about 26,000 hectare with a total production of 530,000 metric tonne per year in Malaysia [1, 2]. Banana bears fruit only once a lifetime, which requires 10-12 months from planting to harvest. In normal cases when the fruit is harvested, the banana tree will be cut, leaving the bottom part of the stem and rhizome untouched for the new tree to grow from it. For every tonne of bananas picked, 100 kg of fruit is rejected and about 4 tonnes of wastes are generated [3]. It means four times of wastes are generated in every cycle of production. Banana wastes range from the rotten fruit, peel, empty fruit bunch, leaves, pseudo-stem, and rhizome. There are many ways to utilize the banana, from fruits till its wastes. The fruits can be eaten raw, cooked, or processed becoming candy or liquor. The rotten fruits and the peels can be processed to feed poultry, pigs, and other animals. The leaves can be used for wrapping food. The pseudo-stems can be processed becoming ropes, crafts, textile, paper and boards. All parts of banana wastes can also be composted as fertilizer, suitable for some vegetables. Banana wastes can also be a potential source of energy. The wastes can be compacted into briquette [4, 5]. The wastes can also be biochemically converted to methane gas with anaerobic digestion [6, 7], or fermented to ethanol [8, 9, 10]. Direct combustion of pseudo-stems and leaves can also generate power [7]. In Malaysia however, farmers only pick banana fruits for food, and fresh leaves for food wrapping. Other portions of banana plant are dumped as wastes. Consequently, farmers often face the problem of disposal of pseudo-stems and these huge stocks are getting accumulated in banana growing areas. Transforming these wastes into energy should be a good consideration for banana culture in Malaysia. It is a big potential for banana wastes to be the feedstock for energy generation and it will solve the country’s agricultural disposal problem in an eco-friendly manner. Very limited studies have been done using banana tree or its peel as feedstock in pyrolysis, which is a promising technology in energy conversion of biomass. Most studies focus on biological conversion (anaerobic digestion) instead. Nevertheless, a group of researchers studied the pyrolysis behaviour of four biomass materials: banana stem, bagasse, babool and castor oil plant [11]. All materials have been pyrolyzed to 650oC. They found out the pyrolytic carbon from banana stem exhibits higher liquid adsorption. Characterization of lingo-cellulosic materials extracted from the banana stem has also been reported [12]. The pyrolytic cellulose, hemicellulose and lignin after the pyrolysis process was characterized. It was suggested that more studies should be done for thermal conversion on banana waste as semi-dry samples of banana leaves presented 77.8% volatile solids, 44% carbon and a heating value of 19MJ/kg with low emission of pollutant gases [3]. The aim of this work is to characterize banana plantation wastes through proximate, elemental, chemical, and thermo-gravimetric analyses, and heating caloric value, in order to evaluate its use as combustible biomass in generating energy and obtaining added value products. However, the study was limited to banana leaf and pseudo-stem only. 2012 National Physics Conference AIP Conf. Proc. 1528, 325-330 (2013); doi: 10.1063/1.4803618 © 2013 AIP Publishing LLC 978-0-7354-1153-1/$30.00 325 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions EXPERIMENTAL ASPECTS Sample Origin and Preparation The feedstock was supplied by a farmer from Pendang, Kedah. A single mature banana tree of 11 months was cut onto a clean canvas to prevent contamination of soil to the sample. The leaves with petiole were dried in air at room temperature for 21 days. The pseudo-stem was oven dried at 105oC until the moisture content of the sample is less than 10% on dry basis. The samples should have moisture content less than 10 mf wt.% to avoid growth of fungus or microorganism [13]. The banana leaves and stem were then manually chopped into smaller pieces so that they could be fed into a shredder. Subsequently, a Retsch cross beater mill with a screen size of 500m was used to reduce the leaves and stem size. Sample Characterization Proximate Analysis The moisture, volatile, and ash contents of the samples were determined through thermogravimetry (in the oven or furnace). The moisture was determined using standard method ASTM E871-82 in a conventional oven. The volatile matter was determined using standard method ASTM E872, and the ash was determined using standard method ASTM D 1102-84 in the furnace. The fixed carbon was determined through the difference of the sum of the others in relation to the total sample. All analyses were performed in triplicate. Elemental Analysis The carbon, hydrogen, nitrogen and sulphur content of the samples were determined in the Perkin-Elmer Series II CHNS/O 2400 Elemental Analyser. The oxygen content was determined through the difference of the sum of the others in relation to the total sample. Heating Value The high heating values (HHV) of the samples were determined using an Adiabatic Bomb Calorimeter (Nenken 1013-B, Japan). All analyses were performed in duplicate. Low heating values (LHV) of the samples were calculated from the higher heating value (HHV) and hydrogen content according to (1) [14]: ࡸࡴࢂࢊ࢘࢟ ൌ ࡴࡴࢂࢊ࢘࢟ െ Ǥ ሺૡǤ ૢࡴȀሻࡹࡶȀࢍ (1) Chemical Analysis The extractives, lignin, holocellulose, alpha-cellulose, and ash content were determined using standard test methods ASTM D1107, D1106, D1104, D1103 and D1102-84, respectively. The hemi-cellulose was determined through the difference of holocellulose and alpha-cellulose. All analyses were performed in duplicate. Thermal Analysis Thermal behaviour of the samples was evaluated through thermo-gravimetric (TG/DTG) analysis. The temperature range used was from room temperature up to 900oC, with nitrogen gas at 30mL/min volumetric flow. The heating rate used was 10oC/min. These analyses were carried out with a computerized Perkin-Elmer 7 Thermogravimetric Analyser. 326 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions RESULTS AND DISCUSSION Tables 1 and 2 show the results of proximate, elemental, chemical analyses, and heating value of banana leaves, and banana stem respectively. TABLE 1. Properties of banana leaves Proximate analysis (mf wt.%) Elemental analysis (mf wt.%) Heating value (MJ/kg) Chemical analysis (mf wt.%) Component / Property Moisture Volatile Matter Ash Fixed carbon C H N S O HHV LHV Holocellulose Alpha-cellulose Hemi-cellulose Lignin Extractives Literature values 11.69 77.8 7.02 44,44.01 6.10 1.36 38.84 19 42.83 25.75 17.08 24.84 9.84 References Measured Standard Method [15] [3] [15] [3,15] [15] [15] [15] [3] [15] [15] [15] [15] [15] 7.34 82.0 7.5 10.5 42.42 4.85 2.68 0.44 49.61 17.7 17.6 49.5 31.7 17.8 39.1 15.5 ASTM E871-82 ASTM E872 ASTM D1102-84 By difference Elemental Analyser By difference Bomb Calorimeter ASTM D1104 ASTM D1103 By difference ASTM D1106 ASTM D1107 TABLE 2. Properties of banana pseudo-stem Component / Property Proximate analysis (mf wt%) Elemental analysis (mf wt%) Heating value (MJ/kg) Chemical component (mf wt.%) Moisture Volatile Matter Ash Fixed carbon C H N S O HHV LHV Holocellulose Alpha-cellulose Hemi-cellulose Lignin Extractives Literature values 8.46,8.57,9.7 4 8.65 36.83 5.19 0.93 43.62 43.25, 50.92 31.27, 50.15, 0.77, 14.98 15.07, 17.44 4.46 References Measured Standard Method [12,16,15] 7.14 ASTM E871-82 [15] [15] [15] [15] [15] [12,16] [15,16] [16,15] [15,16] [15] 80.2 11.0 8.8 37.93 4.46 1.87 0.37 55.37 15.5 15.4 64.2 44.3 19.9 28.7 9.4 ASTM E872 ASTM D1102-84 By difference Elemental Analyser By difference Bomb Calorimeter ASTM D1104 ASTM D1103 By difference ASTM D1106 ASTM D1107 The leaf presented a high volatile solid contents of 82 mf wt.% compared to the stem with 80 mf wt.% volatile solid. Volatile matters in the banana wastes were comparable with other biomass such as rice husk 82.7mf wt.% [17], corncob 79.9 mf wt.% [17], cassava stalk 79.9 mf wt.% [18] and cassava rhizome 77.75 mf wt.% [18]. Both samples demonstrated relatively high ash contents, between 7.5 mf wt.% and 11.0 mf wt.% compared to wood. Carbon, hydrogen, nitrogen and sulphur contents in banana leaves were higher than those in banana stem. However, oxygen content in banana leaves was lower than in banana stem. Comparing with other biomass such as cassava stalk and rhizome; sugar cane; and palm oil empty fruit bunches (EFB) in other literatures, both banana wastes contain lower carbon and hydrogen contents but higher nitrogen, sulphur, and oxygen contents [18, 19, 20]. The high oxygen content of biomass implies high volatile and the high hydrogen content of biomass implies high liquid yields [21]. The calorific heating value of banana leaves (17.7 MJ/kg) and stem (15.5 MJ/kg) are lower than the calorific heating value in palm oil wastes (EFB), which is 19.4 MJ/kg [20]. The lignocellulosic contents of banana leaves are 31.7 mf wt.% for cellulose, 17.8 mf wt.% for 327 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions hemicellulose, and 39.1 mf wt.% for lignin. The lignocellulosic contents of banana stem are 44.3 mf wt.% for cellulose, 19.9 mf wt.% for hemicellulose, and 28.7 mf wt.% for lignin. It shows that banana leaves have lower cellulose and hemi-cellulose but higher lignin components than the banana stem do. The behaviour of a biomass during pyrolysis can be forecasted from knowledge of these ligno-cellulosic components [21]. Thermal Analysis Figure 1 shows the TG and DTG profiles of banana leaves for the temperature range of 30oC to 920oC, with a heating rate of 10oC/min. According to the TG curve, the main pyrolysis reactions including depolymerisation, decarboxylation and cracking, took place over a temperature range of 170oC to 570oC. The TG plot presented in Figure 1 shows that heat propagated into the raw banana leaves and drove off the inherent moisture, which was about 6.51% of the sample weight, at about 170oC. This is comparable to the data in Table 1. At a heating rate of 10oC/min, thermal degradation of raw banana leaves was initiated at approximately 170oC, and the rate was maximum between 300oC and 350oC. At a temperature of about 570oC, the devolatization process the sample was almost complete. The residue as char was left about 25% of the sample weight. The TGA results for banana leaves showed two main regimes of weight loss: the lower temperature regime (170oC - 370oC) could be correlated with the decomposition of hemicellulose and the initial stages of cellulose decomposition, while the upper temperature regime (370oC - 570oC) correlated mainly with the later stages of cellulose decomposition. Lignin thermal decomposition occurred throughout the temperature range of pyrolysis (170oC - 900oC). The DTG curve shows the thermal decomposition of hemicellulose and cellulose occurred at temperatures of around 353oC at a devolatization rate of 0.281-weight loss per minute. FIGURE 1. Thermogravimetric (TG) and derivative Thermogravimetric (DTG) plots for pyrolysis of banana leaves at heating rate of 10oC/min. Figure 2 shows the TG and DTG profiles of banana pseudo-stem for the temperature range of 30oC to 920oC, with a heating rate of 10oC/min. According to the TG curve, the main pyrolysis reactions including depolymerisation, decarboxylation and cracking took place over a temperature range of 150oC to 730oC. The TG plot presented in Figure 2 shows that heat propagated into the raw banana pseudo-stem and drove off the inherent moisture, which was about 1.63% of the sample weight, at about 150oC. This is comparable to the data in Table 2. At a heating rate of 10oC/min, thermal degradation of raw banana pseudo-stem was initiated at approximately 150oC, and the rate was maximum between 300oC and 350oC. At a temperature of about 430oC, the devolatization process the sample was almost complete. The residue as char was left about 39% of the 328 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions sample weight. The DTG curve shows the thermal decomposition of hemicellulose and cellulose occurred at temperatures of around 325oC at a devolatization rate of 6.386-weight loss per minute. FIGURE 2. Thermogravimetric (TG) and derivative Thermogravimetric (DTG) plots for pyrolysis of banana pseudo-stem at heating rate of 10oC/min. CONCLUSIONS The banana leaf sample showed greater volatile matter, elemental carbon and calorific value contents than the banana stem sample. Both nitrogen and sulphur contents in banana stem were lower than in banana leaf. Please note that the nitrogen and sulphur contents were relatively low for both banana leaf and banana stem, contributing to a reduction in pollutant gas emissions during combustion. The alpha-cellulose and lignin contents in banana stem are greater than in banana leaf sample. The thermal behaviour of both samples showed similarity but distinct in proportions. The physical and chemical properties of the samples were similar to the other biomass already used for the purpose of generating energy and obtaining value added products through combustion and pyrolysis processes. This indicates their potential for reuse. These thermochemical conversion processes result in significant volumetric reduction in wastes. Consequently, it helps reducing environmental impact generally caused by its disposal. ACKNOWLEDGEMENTS The authors wish to thank Universiti Sains Malaysia for providing financial supports under research grant (1001/PFIZIK/814087), short term grant (304/PFIZIK/6310073), and short term grant (304/PFIZIK/6310087). The authors also would like to thank UNISEL for providing educational scholarship and Muhammad Sabki Bin Yusoff for supplying the banana wastes, which is the primary material in this study. 329 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions REFERENCES 1. S. Mekhilef, R. Saidur, A. Safari and W.E.S.B. Mustaffa, Renewable and Sustainable Energy Reviews 15 (7), 3360-3370 (2011). 2. C. S. Goh, K. T. Tan, K. T. Lee, and S. Bhatia, Bioresource Technology. 101 (13), 4834-4841 (2010). 3. N. Sellin, O. Souza, S. H. W. Medeiros, and R. K. S. Afuso, presented at the Third International Symposium on Energy from Biomass and Waste. Venice, Italy, 2010 (unpublished). 4. P. Wilaipon, American Journal of Applied Sciences. 6 (1), 167-171 (2009). 5. H. Lee and Z. Smith, Feasibility of Biomass Fuel Briquettes from Banana Plant Waste. (Engineers Without Borders Greater Cincinnati Professional, Cincinnati, 2011). 6. T. M. Khan, C. Maurer, D. Argyropoulos, M. Brule and J. Muller, presented at the Tropentag International Research and Food Security, Natural Resource Management and Rural Development. Hamburg, Germany, 2009 (unpublished). 7. J. Y. Tock, C. L. Lai, K. T. Lee, K.T. Tan and S. Bhatia, Renewable and Sustainable Energy Reviews. 14 (2), 798-805 (2010). 8. A. B. M. S. Hossain, S. A. Ahmed, A. M. Alshammari, F. M. A. Adnan, M. S. M. Annuar, H. Mustafa and N. Hammad, African Journal of Microbiology Research 5 (6), 586-598 (2011). 9. S. Graefe, D. Dufour, A. Giraldo, L. A. Munoz, P. Mora, H. Solis, H. Garces and A. Gonzalez, Biomass and Bioenergy. 35 (7), 2640-2649 (2011). 10. H. I. Velasquez-Arredondo, A. A. Ruiz-Colorado and S. De Oliveira Junior, Energy. 35 (7), 3081-3087 (2010). 11. S. Manocha, J. Bhagat, M. Patel, N. Patel and L.M. Manocha, in the Adsorption Science and Technology of the Second Pacific Basin Conference. (Brisbane, Australia, 2000), pp. 727-731. 12. A. L. S. Pereira, D. M. Nascimento, E. M. S. Cordeiro, and J.P.S. Morais, in 7th International Symposium on Natural Polymers and Composites 2020. (Gramado, Brazil, 2010), pp. 1077-1079. 13. F. Sulaiman and N. Abdullah, Energy, 36 (5), 2352-2359 (2011). 14. Phyllis, Database for Biomass and Waste, (Energy Research Centre, Netherlands, 2005). 15. K. Bilba, M. A. Arsene and A. Ouensanga, Bioresource Technology 98 (1), 58-68 (2007). 16. J. L. Guanimaraes, E. Frollini, C. G. da Silva, F. Wypych and K. G. Satyanaryana, Industrial Crops and Products. 30 (3), 407-415, (2009). 17. K. Raveendran, A. Ganesh and K. Khilar, Fuel. 74 (12), 1812-1822 (1995). 18. A. Pattiya, J. O. Titiloye and A. V. Bridgwater, Asian Journal on Energy and Environment. 08 (02), 496-502 (2007). 19. R. Xu, L. Ferrante, C. Briens and F. Berruti, Journal of Analytical and Applied Pyrolysis. 91 (1), 263-272 (2011). 20. N. Abdullah, H. Gerhauser and F. Sulaiman, Fuel. 89 (8), 2166-2169 (2010). 21. P. Basu, Biomass Gasification and Pyrolysis: Practical Design and Theory. (Elsevier Inc., Amsterdam, 2010) 330 Downloaded 16 Jul 2013 to 103.1.70.232. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://proceedings.aip.org/about/rights_permissions