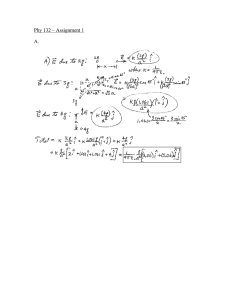
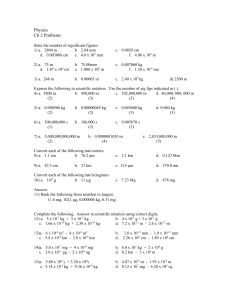
2016 SCH3U Introduction to Chemistry Quiz . Name________________ Date_________________ 1. Determine the number of significant digits in the following: (3) a. 40000g ____________ 71 b. 1.0000 x10 ____________ c. 0.000022 ____________ 2. Complete the following: (3) a. 30.5 g - 101.1 g = _________ b. 5.000g + 24 g= _________ 3. 4. 5. 6. 7. c. 64g 0.21 = _________ Convert each to scientific notation: (3) a. 0.100024 ___________ b. 1000.00 ___________ c. 45 00000 ___________ Convert each into decimal form: (3) a. 2.7 x10 -6 ___________ 4 b. 7.99 x10 ___________ 1 c. 95 x10 ___________ Convert the following into percent uncertainties. Round your answer to one sig dig.(2) a. 2.70 0.05 cm = ___________ b. 11.02 0.8 cm =___________ Convert the following into absolute uncertainties: (2) a. 13.7 6% =____________ b. 25 s 20% = ____________ Calculate the answer with the absolute uncertainty: (4) a. (32.0 0.1 mL ) +(10.0 0.5mL) = b. (5.2 0.1 m) x (10.2 0.5m) = 8. Explain what is meant by the following terms when applied to scientific measurements a. Precise (2) b. Accurate (1) 9. For each of the following diagrams express the reading showing the quantity and uncertainty. (6) 10. Label each piece of laboratory equipment pictured below(4)