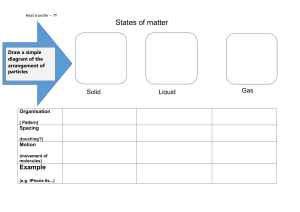
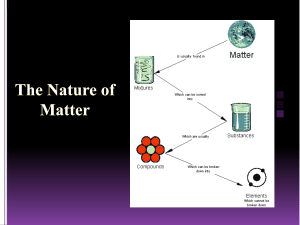
Republic of the Philippines Department of Education Region VIII Division of Samar STA. MARGARITA NATIONAL HIGH SCHOOL School ID: 303629, Sta. Margarita, Samar Name :_____________________________________ Grade: _______________________________ Section:____________________________________ Date: _________________________________ LEARNING ACTIVITY SHEET IN SCIENCE 8 Quarter 3 – Module 2 Atoms and Molecules What I Need to Know At the end of this module, you should be able to: 1. Explain physical changes in terms of the arrangement and motion of atom and molecules. (S8MT – IIIc-d-9) What I Know (Pre-Test) Instructions: Read and answer the questions below. Write the letter of the correct answer in your paper. 1. Which of the following does NOT prove that the molecules of a material are always moving? A. Aroma of boiling coffee B. Evaporation of water C. Soil particles carried by the water D. Sweetness of sugar 2. Which of the following is a mixture? A. Distilled water B. Fruit sugar C. Soy sauce D. Table salt 3. Why could you hardly break a stone even if much force is applied to it? A. are negligible B. compactly arranged with one another C. loosely bound together D. very far from one another 4. When a drop of ink was put into a glass of water, its tendency is to spread out. This is because the atoms of ink __________________. A. are not moving B. are compact and rigid C. are in random motion D. have distinct characteristics 5. What is formed if you mix water and soy sauce? A. a 1-phase system B. a 2-phase system C. a heterogeneous system D. a compound 6. Which of the following is a substance? A. Copper B. Padlock C. Pin D. Solder 7. How does a mixture different from a substance? A. In mixture it keeps its characteristics B. Mixtures are found in nature C. Mixtures have 2 or more components D. Solids, liquids and gases can form mixture 8. The only mixture that looks like only one chemical composition in physical appearance. A. Colloid B. Compound C. Solution D. Suspension 9. Which of the following has one kind of atom? A. Carbon dioxide B. Gold C. Iron oxide D. Water 10. Which of the following is a substance? A. Copper B. Key C. Padlock D. Solder Lesson 1: Physical Changes in Terms of the Arrangement and Motion of Atoms What I Need to Know At the end of the lesson, you are expected to: 1. Explain the arrangement and motion of atoms. Activity 1: Give me space Objective: After doing this activity, you should be able to: 1. Show and describe the spaces between atoms. Materials: Sand Marbles / Plastic beads transparent glass water medicine dropper Procedure: 1. Fill a glass with marbles or plastic beads until you cannot add more. Then pour grains of sand into the same container. 2. Next, fill another glass with as much water as it can hold. Use a medicine dropper to add the last few drops. Then gently drop few grains of sand into the water. 3. Shake the mixture. Observe again what happens. Q1. Why can the glass container accommodate the sand when it cannot accommodate more marbles? Q2. Does the water spill? Why? Q3. What happens when you pour slowly the sand into the glass of water? Explain. Activity 2: What is Matter made of? Objective: After this activity, you should be able to: 1. Describe the arrangement of atoms in liquids. Materials: a glass of water, medicine dropper, food coloring or ink Procedure 1. Place a drop of ink or food coloring into the glass of water. 2. Do not disturb the glass of water. Observe what happens to the color of the water. 3. While the particles of ink or food coloring are in motion, record the time consumed. 4. Note the time taken to travel the particles of the ink or food coloring. Table 1. Relationship between motion and time Time (t) seconds Observations Questions: Q1. What do you observe? __________________________________________________ Q2. Describe how the color spreads in the water. _________________________________ Q3. Why does the water change its color? _______________________________________ Q4. Did the color spread faster in the water? _____________________________________ Q5. What property will explain based on what you have observed? ___________________ What Is It Pure Substance Matter are often classified into pure substance and mixtures. Substance are often a component or compound. Matter endure either physical or chemical change. Physical change occurs when a substance changes its appearance without changing its composition. Phase change is the process of changing from one physical state to another. While chemical change occurs when a substance is transformed into another substance. Substance is a homogeneous material consisting one particular kind of matter. Homogeneous mixture is a substance that exist in one phase of matter. Mixture is composed of two or additional pure substance. It is a material consisting two or more kinds of matter each of which retains its own characteristic. Mixture can be homogeneous mixture having uniform in appearance or heterogeneous having non- uniform in appearance. An atom is that the smallest particle of a component that has all the properties of the element. Atom of the most elements have the ability to combine with other atoms. Different elements have different properties because the combining atoms are different and the way the atoms are joined together are different. Atom is made even smaller particles. SOLID LIQUID GAS SOLID SOLID Figure 3. Particles of Solid, Liquid, and Gas Molecules of solids are compact. They have small spaces between them. This prevents from moving freely. They vibrate in fixed position. Solids have definite shape and volume because the particles are closely packed. They vibrate a little but in fixed positions. The particles cannot move around. The particles of solid held together by strong force. The volume of solid is definite In liquids, molecules move slowly and are closer together. Liquids have an exact volume and however assume the form of the container. Their molecules have farther spaces. The attraction between particles is stronger than those of gases. The particles move and change position. Gas has no definite shape and volume. It spreads to fill up whatever space is available. Gas particles move very fast and are very far from each other. What I Have Learned Activity 3: Try Again! Instructions: Below are different materials. Identify whether it is a substance or a mixture. Put a check in the appropriate column. Chemical System Substance Mixture 1.Salt solution 2.Brown sugar 3.alcohol 4.Tawas 5.Milo in hot water 6.Salt 7.water 8.Iron 9.Softdrink 10.Charcoal Q1. When can you say that a chemical system is a substance or a mixture? What I Can do Activity 4: THIS IS IT! 1. Cut – out pictures of different objects like drinks, balloons, medicines lotions, beauty products from old magazines. Identify which are mixtures and substance. Cite importance of mixture in your daily life. Lesson 2: Physical Changes in Terms of the Arrangement and motion of Molecules What I Need to Know Objectives: At the end of lesson, you are expected to: 1. explain physical change in terms of the arrangement and motion ofmolecules. What’s New Activity 1: What is Matter made of? Objective: After performing the activity, you should be able to: 1. Explain how these observed situations or events give evidence that matter is made up of tiny particles. Materials: clean tap water Transparent glass Spoon 10 ml alcohol / 10 tablespoons of alcohol 50 ml water / 1 cup of water Procedure: 1. Use a clean transparent glass, pour alcohol up to 20 ml (10 table spoon). 2. Measure 50 ml of water. 3. Add the 50 ml water to the alcohol and mix through. Observe the resulting solution. (Caution: Do not taste the solution) Q1. What is the appearance of the resulting mixture? _____________________________________________ Q2. Think about the alcohol and water as made as tiny particles. Give your reasons for the observations you made in Q1. ______________________________________________________________________________ Q3. What is the volume of alcohol and water mixture? _____________________________________________ Q4. Is there a change of volume after mixing these two liquids? _____________________________________ What Is It Molecules are composed of Atoms The existence of matter in a solid, liquid, or gaseous phase enables us to infer the structure of matter. Scientists theorized that molecules are further composed of particles, even smaller called atoms. In 1805, John Dalton an English scientist, proposed the atomic theory of matter. This theory states that all matter consists of very tiny particles, the atoms. In some cases, a molecule is made up of only one atom. An example of this is a molecule of water composed of two atoms of hydrogen and one atom of oxygen (H2O.) The molecules are constantly moving. Molecules of liquids and gases spread from where there are more molecules to places where there are few molecules. This process is known as diffusion. Matter can be classified as mixture, which can be broken down by physical means, or a pure substance, which cannot be broken down physically into simpler substance. There is some mixture specially solution which are substance-like because it appears to have one chemical composition only like seawater and vinegar. What I Have Learned Activity 2: Answer Me Objective: To be able to identify substance and mixture. Instructions: Identify the given chemical system as substance or mixture. Write your answer on the space provided. Number one has been done for you. Chemical System Substance or Mixture 1.sugar Substance 2.ice cream 3.paper 4.alcohol 5.salt 6.rice grain 7.bottled water 8.Peanut butter 9.halo-halo 10.vinegar Summary Matter has mas and it occupies space. Matter can be classified into solids, liquids, and gases. Solids are substance with a definite volume, but the assume the shape of the container. Gases are substance with neither a definite volume nor a definite shape. Matter is composed of particles called molecules. Molecules in solids are arranged farther apart. The molecules in liquids are arranged farther apart than in solid. The molecules in gasses are arranged still farther apart than in liquids Molecules are composed of smaller parts called atoms. Spaces exist between molecules. Molecules are in constant motion. Molecules in gases move fast. In liquids they move slower, and in solid, it moves slowest. Evaporation is the process by which liquid change to gas. Condensation is the process in which a gas is change to liquid. Melting the process in which a solid will change to liquid. Freezing is the process in which a liquid will change to solid. Mixture has no definite properties Substance is a homogeneous material consisting of one particular kind of matter. Homogeneous mixture when it has uniform composition. Heterogeneous mixture when its components and properties are not distributed evenly. Chemical Change when substance change its appearance, occurs when it transforms into another substance having different set of properties. Physical change when substance changes its appearance. Assessment (Post-Test) Instructions: Read and answer the questions below. Write the letter of the correct answer in your paper. 1. When you were able to observe the sweet smell of ripe fruit from the dining table, you are aware that a process had occurred. What do you call this natural process? A. Absorption B. Assimilation C. Diffusion D. Digestion 2. Molecules constantly move from one place to place. In which of the following is the movement of the molecules fastest? The molecules of_______________________. A. a gas in another gas B. a liquid in another liquid C. a solid in a liquid D. a solid in another solid 3. A mixture that has the same appearance and composition throughout? A. Colloidal B. Heterogeneous C. Homogenous D. Tonic 4. At the right is a diagram of a molecule of water as seen under the electron microscope. What makes up a molecule of water? A. An atom of hydrogen and three atoms oxygen. B. An atom of oxygen and two atoms of hydrogen. H O H C. An atom of hydrogen and three atoms oxygen. D. Three atoms of hydrogen and two atoms of oxygen. 5. Which of the following is an example of a heterogeneous mixture? A. Halo - halo B. Metal alloy C. Sea water D. Vinegar 6.Which of the following shows physical change? A. Boiling an egg B. Boiling of water B. Burning of wood D. Digesting of food 7. Mixing 20 grams of sugar and 50 mL of water results a volume of less than 70 mL. What explains this result? A. Sugar dissolves in water. B. There is an error measuring the amount of sugar and water. C. Water has tiny particles with spaces between them, allowing sugar particles to fit in. D. Water has close particles with no spaces between them, sugar particles cannot fit in. 8. In the three phases of matter, which has the strongest force of attraction? A. Freezing B. Gas C. Liquid D. Solid 9. When liquid turns into solid, which of the following physical change occurs? A. Condensation B. Deposition C. Freezing D. Sublimation 10. Which of the following is not a mixture? A. Mayonnaise B. Shampoo C. Softdrink Prepared by: MRS. MARIA CRISTINA C. DELMO Subject Teacher, Science 8 Contact Number: 0916-354-4792 (globe)/0909-358-2167 (smart) FB: Maria Cristina Delmo Gmail: macristinadelmo2187@gmail.com D. Tawas 3D ATOMIC MODEL TASK: Create a 3-D model of an atom of assigned element following the Bhor’s Atomic Model. Rubric for 3 D Atomic Model Criteria 10 points 7 points 5 points creativity Exceptionally degree of student creativity is displayed in flexibility of thought and originality Only few areas of the ATOMIC MODEL the student creativity Minimal creativity or ATOMIC MODEL is a recreation of a existing image Minimal creativity or ATOMIC MODE is a recreation of a preexisting image The organization of the ATOMIC MODEsynthesizes independent parts into a singular work of art that unifies and balances. The organization of the ATOMIC MODEsynthesizes all but a couple areas into a singular work of art that unifies and balances The organization of theATOMIC MODEsynthesizes all but a few areas into a singular areas into singular work of art that unifies and balances. The ATOMIC MODE does not unify and appears disjointed ATOMIC MODE is seamlessly assembled and completely covered. Pieces are precisely cut and well glued down. Much time and effort went into the planning and designing of the ATOMIC MODE.It is clear the student worked at home. ATOMIC MODE is well assembled with only few pieces that are not well cut out or glued down. ATOMIC MODE is well assembled with only several pieces that are not well cut out or glued down Haphazard Craftsmanship Student could have put in more time and effort at home. Class time was not always used wisely, but student did do some Addition at home. Time was not used wisely. Student put in no additional effort organization craftsmanship Time and Effort Example: 3 points What is Atomic Model? Is a 3D concept of atom. Materials: Cup of cans (takip ng mga bote, softdrinks) Marble o dyolens or styro foam balls Stick glue Colored paper Scissor Glue Wire or alambre Note: Para sa stand nito maaari kayong gumamit ng isang piraso ng maliit na flywood o kahit anong bagay na mabigat upang ito ay tumayo. Huwag kalimutang ilagay ang pangalan ng atom. Paano Gawin: Pumili ng isang element sa atoms o molecules na bubuuin mo na model. Narito ang mga halimbawa sa ibaba..maaaring magresearch sa internet sa mas makahanap pa ng ibang halimbawa ng atoms na maaaring gawing proyekto. water IXTURES AND SUBSTANCE INSTRUCTION: Cut – out pictures of different objects like drinks, balloons, medicines,lotions, beauty products from old magazines. Identify which are mixtures and substance. Cite importance of mixture in your daily life. Republic of the Philippines Department of Education Region VIII Division of Samar STA. MARGARITA NATIONAL HIGH SCHOOL School ID: 303629, Sta. Margarita, Samar Name :_____________________________________ Grade: _______________________________ Section:____________________________________ Date: _________________________________ LEARNING ACTIVITY SHEET IN SCIENCE 8 Quarter 3 – Module 2 Atoms and Molecules Assessment Directions: Choose the letter of the correct answer and write it in your notebook. 1. Which statement is TRUE about the aspects of the particle model of matter? A. Matter is made up of bigger particles. B. Particles of matter do not attract each other. C. Particles of matter are not moving all the time. D. Particles of matter have spaces between them. 2. Which would not change when an orange juice is poured from a can into a drinking glass? A. the color of the orange juice B. the shape of the orange juice C. the taste of the orange juice D. the volume of the orange juice 3. What happens to shape and size of the stone if you put it in a smaller container? A. A stone keeps its original shape and volume. B. A stone keeps its original shape but not its volume. C. A stone keeps its original volume but not its shape. D. A stone changes its original volume and its shape. 4. Why can many balloons be filled from one small tank of helium? A. The particle of helium gas in a balloon is locked in. B. The particles of helium gas in a balloon are far apart. C. The particles of helium gas in a balloon are slightly apart. D. The particles of helium gas in a balloon are very closed to each other. 5. When you poured the honey to a tablespoon it flows easily, what happens to the 6. 7. 8. 9. particles of honey? A. The particles slide past one another. B. The particles come close together and vibrate. C. The particles are moving far apart and independent of one another. D. The particles are locked in place and vibrate independently to each other. Imagine inflating a balloon. Would anything happen to the shape and size of the balloon? Which of the following statements support the correct idea? A. Only its shape increases. B. Only its volume increases. C. The shape of the balloon increases and also its volume. D. Only the shape of the balloon increases and nothing happens to its volume. Why does cooking oil take the shape of its container? A. The cooking oil is a liquid that has a definite volume only. B. The cooking oil is a liquid that has a definite shape and volume. C. The cooking oil has a definite volume but does not take the shape of the D. container. D. The cooking oil is a liquid that has a definite volume but takes the shape shape of the container How would you explain the images of the solid particles below? A. Unnoticeable space between particles. B. There are lots of free spaces between them. C. There’s only little free space between them. D. Particles can move fast one another at the same time. How would you explain the distances of liquid particles? A. There’s only little free space between them. B. There’s unnoticeable space between particles. C. There are lots of free spaces between them. D. Particles kept on moving at any direction from one place to another. 10. One property of the microscopic behavior of matter is the ability of the particles to move. Rank solid, liquid, and gas in order of the particles speed from highest to lowest. A. Gas -----Solid -----Liquid C. Gas -----Liquid ----- Solid B. Solid ----Gas ----- Liquid D. Liquid ---Gas ------- Solid Prepared by: MRS. MARIA CRISTINA C. DELMO Subject Teacher, Science 8 Contact Number: 0916-354-4792 (globe)/0909-358-2167 (smart) FB: Maria Cristina Delmo Gmail: macristinadelmo2187@gmail.com